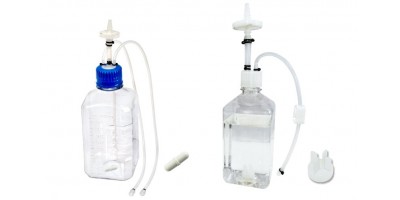
AseptiMix™ VB Vented Bottle Mixer Assemblies
mdi AseptiMix™ VB are gamma-sterilized vented bottle mixer assemblies, suitable for mixing, safe transfer, and storage of biopharmaceutical products and reagents. The assembly does not require any additional hardware and can directly be placed on a magnetic mixer for mixing with a stir bar or impeller placed inside.
The stir bar/impeller inside the mixer assembly has wide chemical compatibility and it ensures the proper mixing of solution without any particle shedding.
These assemblies are fitted with a self-supporting lightweight, sterilizing grade 0.2μm PVDF vent filter to prevent ingress of microorganisms during filling and removal of high-value products.
- Mixing, storage and transfer of cell culture media and buffers
| Materials of Construction | |||||
| Cap | Polypropylene | ||||
| Inlet Tube | Platinum cured silicone | ||||
| Bottle |
|
||||
| Dip Tube | Platinum cured silicone | ||||
| Vent Filter |
|
||||
| Stir Bar | PVDF | ||||
| Impeller | Polypropylene | ||||
| Assurance | |||||
| 100% Integrity Tested | Pressure Leak Test | ||||
| Toxicity | Passes Bioreactivity test, In Vivo, as per USP <88> for Class VI plastics | ||||
| Particle Release | Complies with USP <788> test for particulate matter in injections | ||||
| Endotoxin Testing | Aqueous extracts exhibit <0.25 EU/ml as established by Limulus Amebocyte Lysate (LAL) Test as per USP <85> | ||||
| Quality Management System | mdi AseptiMix VB vented bottle mixer assemblies are well-designed products with in-built quality assurance. ISO-9001:2018 Certified Quality Management System, careful selection of raw materials, validated production processes and testing procedures based on international standards and guidelines such as CFR, PDA, and ASTM, ensure the manufacture of consistently high quality assemblies |
||||
| Extractables | Passes NVR test as per USP <661> | ||||
| Sterility | mdi AseptiMix VB vented bottle mixer assemblies are sterilized by gamma irradiation to provide a sterility assurance level of 10-6. The sterilization process has been validated as per ISO 11137-2 which includes dose verification, dose mapping, and quarterly dose audits. The sterilization dose of 25 kGy has been substantiated through the careful definition of the test samples, bioburden testing of multiple lots of the selected test samples, calculation of verification dose, and sterility testing. |
||||
Customization
mdi AseptiMix™ VB vented bottle mixer assemblies can be customized to suit user requirements in terms of tubing, fittings, end connections, and connectors.
| Datasheet | Download |