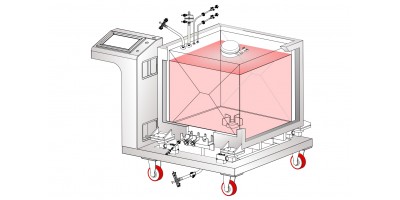
AseptiMix™ MI 3D Single Use Mixer Bags
MDI AseptiMix™ MI 3D mixer bags provide validated and reliable mixing solutions for biopharmaceutical process requirements such as mixing of
- Cell culture media
- Process intermediates
- Sterile buffers with wide ranging pH
- Formulations
MDI AseptiMix™ MI single use mixer bags are designed for uniform and fast mixing. The impeller is located inside the AseptiMix™ MI mixer bags which is rotated with the help of a magnetic drive. These are available for volumes upto 3000 liters.
Powder Ports
The AseptiMix™ MI mixer bag is also available with 4” and 8” sanitary flange powder ports for powder-to-liquid mixing.
- Custom designed to suit user specific process applications
- Impeller for uniform and easy mixing
- Available with 4” and 8” sanitary flange powder port for powder-to-liquid mixing
- Easy inlet and outlet quick connections
- High barrier properties for protection of product molecule, product pool and media components
- 100% integrity tested
- Powder to liquid mixing
- Liquid to liquid mixing
| Materials of Construction | |||||||||||||||||||||||||||||||||
| Impeller | Polypropylene | ||||||||||||||||||||||||||||||||
| Bag Film | AseptiFlex-D film type FBG-1 | ||||||||||||||||||||||||||||||||
| Tubing |
|
||||||||||||||||||||||||||||||||
| Dimensions | |||||||||||||||||||||||||||||||||
| Bag Size |
|
||||||||||||||||||||||||||||||||
| Assurance | |||||||||||||||||||||||||||||||||
| Bacterial Endotoxin | Aqueous extracts exhibit < 0.25 EU/ml as established by Limulus Amebocyte Lysate (LAL) Test as per USP <85> | ||||||||||||||||||||||||||||||||
| Fiber Release | Passes test as per USP and comply with USFDA 21 CFR Part 210.3(b)(6) for fiber release | ||||||||||||||||||||||||||||||||
| Particle Release | Complies with USP <788> test for particulate matter in injections | ||||||||||||||||||||||||||||||||
| Extractables with WFI | Does not affect the quality of Water for Injection (passes tests as per USP <661>) | ||||||||||||||||||||||||||||||||
| Sterilization | Gamma Sterilizable upto 50 kGy | ||||||||||||||||||||||||||||||||
| Sterility | The gamma sterilization process has been validated as per ISO 11137 to ensure a sterility assurance level(SAL) of 10-6 | ||||||||||||||||||||||||||||||||
| Biosafety | Passes the Biological Reactivity Tests, In Vivo for Class VI plastics as described in USP <88>. | ||||||||||||||||||||||||||||||||
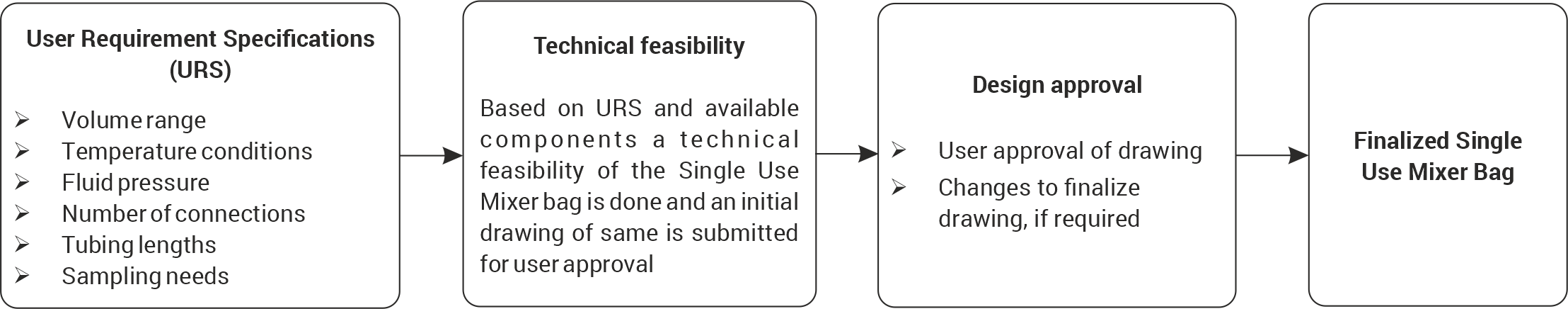
These AseptiMix™ MI mixing systems can be customized to suit user requirements regarding tubing sizes, type of inlet ports, sampling ports, and position and type of drain ports. A technical feasibility of the required design is established based on available components and an initial drawing is proposed. Products prototyping and final approval leads to customized mixer bag realization.
| Datasheet | Download |
AseptiMix™ MI is made from AseptiFlex™-D film offering multiple advantages such as:
- Uniform and fast mixing
- Customized to user requirements
- Very low extractable profile for low ‘Product’ risk