Home » Products » Products » Single Use Systems » Bioreactors »
AseptiWave™ Bioreactors
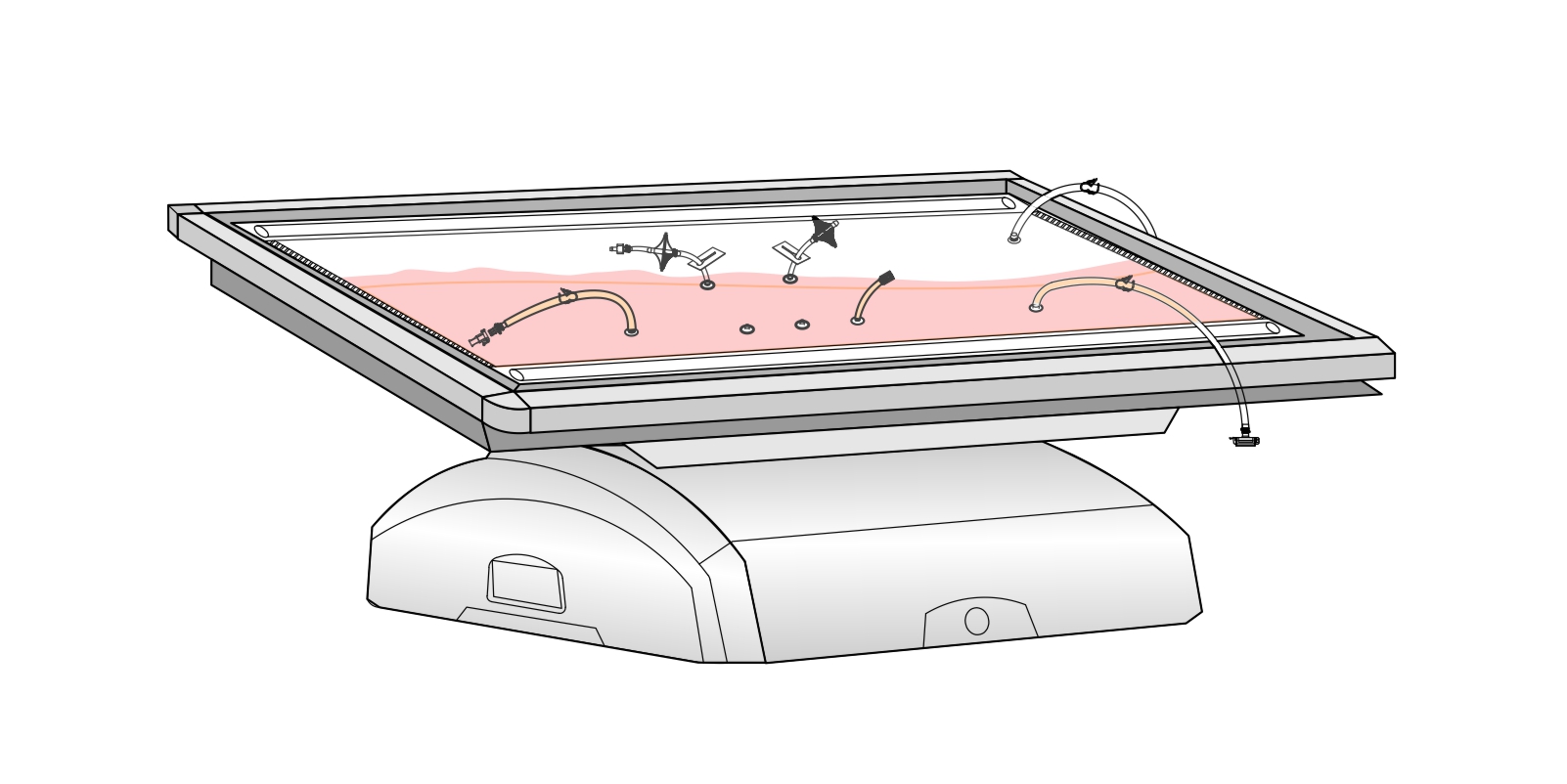
MDI AseptiWave™ single use bioreactors are designed for efficient culture of different type of cells including Mammalian cells, Plant cells, Insect cells, Microbial cells, Stem cells etc.
These are available gamma sterilized in multiple sizes, from 2 liters for clone selection and media optimization labs to 50 liters for process development and GMP production of biopharmaceuticals.
- Cell Culture
- Expansion of anchorage dependent cells such as epidermal and connective tissue cells
- Small scale expansion of stem cells
- Inoculum propagation
- Development and manufacture of:
- Therapeutic protein
- Monoclonal Antibodies (mAbs)
- Expansion of CART cells for cell therapy
| Construction | ||
| AseptiWave Bioreactor Film | AseptiFlex I | |
| BioVent Gas Filter | Hydrophobic Filter Media | |
| Specifications | ||
| Size | Minimum Culture Volume* | Maximum Culture Volume |
| 1L | 150ml | 500ml |
| 2L | 300ml | 1L |
| 5L | 500ml | 2.5L |
| 10L | 1L | 5L |
| 20L | 2L | 10L |
| 22L | 2L | 10L |
| 50L | 5L | 25L |
| 100L | 15L | 50L |
| 200L | 30L | 100L |
| *The minimum culture volume specified is for AseptiWave™ Bioreactors with pH and DO sensors. It might be possible to work with lower culture volumes for AseptiWave™ Bioreactors without sensors. However, it is recommended to have culture volumes above the minimum specified volumes for applications that require high agitation. |
||
| Operational | ||
| Sterilization | AseptiWave bioreactors are Gamma Sterilizable up to 50 kGy. | |
| Assurance | ||
| Bioburden | Bioburden level is < 1000 cfu/device as per ISO 11737-1:2018. | |
| Fiber Release | Passes test as per USP and complies with USFDA Title 21 CFR Part 210.3(b)(6) for fiber release. | |
| Particle Release | Complies with USP <788> test for particulate matter in injections. | |
| Endotoxin Testing | Aqueous extracts exhibit <0.25 EU/ml as established by Limulus Amebocyte Lysate (LAL) Test as per USP <85>. | |
| Extractables | The Extractable profile is available as per BioPhorum Best Practices Guide for Extractable Testing of Polymeric Single Use Components used in Biopharmaceutical Manufacturing. | |
| Sterility | The gamma sterilization process has been validated as per ISO 11137 to ensure a sterility assurance level (SAL) of 10-6. | |
| Biosafety | Passes Biological Reactivity test, In-Vivo, as per USP <88> for Class VI plastics. Passes the Biological Reactivity Tests, In Vitro for Cytotoxicity as described in USP <87>. |
|
| File Type | Download |
|---|---|
| Datasheet | Download |
Have questions or need assistance?
Our team is here to help! Contact us today, and we’ll get back to you as soon as possible.