Polysaccharides are large, complex carbohydrate macromolecules made up of long chains of monosaccharide units linked by glycosidic bonds.
Peptidoglycans, lipopolysaccharides, capsules, and exopolysaccharides are among the many different types of macromolecules that make up bacterial polysaccharides. These substances serve as crucial virulence factors (e.g. Haemophilus influenzae, Neisseria meningitidis and Streptococcus pneumonia) as well as structural cell-wall components (e.g. peptidoglycan).
Since free polysaccharides cannot connect to the major histocompatibility complex molecules, they cannot activate T-helper cells, making them T-cell independent antigens. However, polysaccharides can elicit antibody responses by activating B cells and while in adults T-independent responses to free polysaccharides antigens are effective, those of newborns and young children with underdeveloped immune system are only weak. Influenzae, Meningitidis and Pneumonia, and other bacterial illness based on polysaccharides are still common around the world and childhood immunization is required because young children are especially vulnerable to them.
Polysaccharide conjugate vaccines
The haptenic polysaccharide molecule is changed into a T-dependent antigen that is highly effective in triggering an immunological response in newborns and young children by covalently attaching a carrier protein to it. This process is used to create polysaccharide conjugate vaccines against Haemophilus influenzae, Neisseria meningitidis and Streptococcus pneumonia.
Polysaccharide Conjugate Vaccine Manufacturing
For the production of polysaccharide conjugate vaccines, two key components are manufactured separately, the polysaccharide antigen and the carrier protein.
The polysaccharide is obtained by cultivating specific bacteria, harvesting their capsular material, and purifying the polysaccharide using filtration and chromatography to ensure high purity. Simultaneously, the carrier protein such as tetanus toxoid (TT) or diphtheria toxin (CRM197) is produced by inserting its gene into a microbial host like E.coli, followed by fermentation, extraction, and purification.
Both components are then chemically linked in a conjugation step to form the polysaccharide conjugate vaccine, which elicits a stronger and long-lasting immune response.
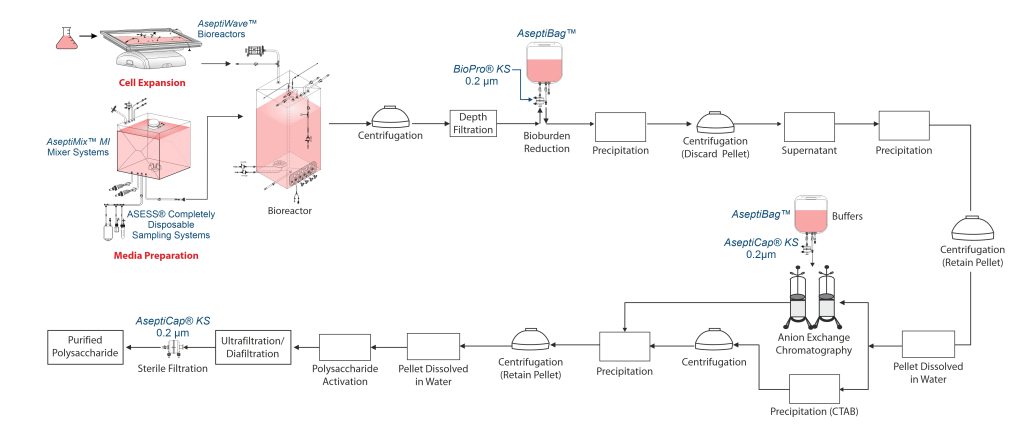
Polysaccharide Manufacturing Process
Pathogenic organisms like Haemophilus, Pneumococcus and Neisseria are used for manufacture of Polysaccharide Conjugated Vaccines (PCV). This requires culture workers to be vaccinated.
The process starts with microbial culture of a specific microorganism in a bioreactor, where media is charged into the bioreactor and sterilized for few hours and then inoculated with bacteria (Haemophilus influenzae, Neisseria meningitidis and Streptococcus pneumonia). Efficient oxygen transfer and silicone anti-foams are used for culture and polysaccharides are secreted into the media during bacterial growth. DOC, a mild detergent, may be added at the stationary phase to enhance secretion.
After microbial culture, cells are removed via centrifugation followed by depth filtration, reducing NTU to 60. The clarified harvest is then passed through a sterilizing-grade filter/ bioburden reduction filter for bioburden control.
Purification
Precipitate removal is done in three steps. First, small molecular impurities are removed by adding few chemicals (0.4% v/v of ethanol in 5% Na-acetate), followed by centrifugation to discard pellet. Next, the polysaccharide is precipitated using similar chemicals(0.75% v/v of ethanol in 7.2% Na-acetate), and the pellet is retained. This is then dissolved in water and treated with 1.5% CTAB to remove about 95% of nucleic acids and 90% of protein contaminants. This is followed by centrifugation and then the resulting polysaccharide pellet is collected.
The precipitate is dissolved in 0.25 M NaCl solution, then clarified using depth filtration. Additionally precipitation of polysaccharide is carried out using 0.75% v/v ethanol, which finally dissolved in water post centrifugation.
Chromatography
Some polysaccharides such as the ones from pneumococcus strains 7F, 14 AND 33F, resist CTAB precipitation. These are purified by anion-exchange chromatography to remove proteins and nucleic acids.
Ultrafiltration/Diafiltration (UF/DF)
Some polysaccharides such as Polyribosylribitol phosphate (PRP) from H. influenzae cannot be directly conjugated to tetanus toxoid. The PRP (1000kDa) is reduced to 250 kDa using carbonate buffer and then activated with adipic acid dihydrazide (ADH) via cyanogen bromide.The unreacted components are removed via UF/DF. The resulting PS-ADH complex is aseptically filtered through a sterilizing grade 0.2µm filter, and then conjugated to a carrier protein such as tetanus toxoid (TT) or diphtheria toxin (CRM197).
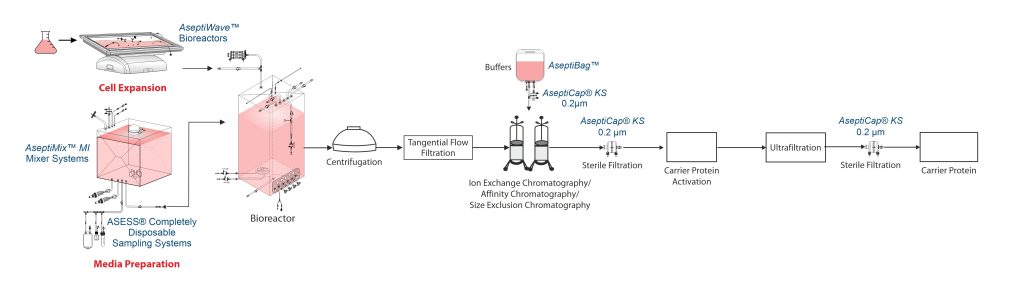
Carrier Protein Manufacturing Process
Carrier proteins like tetanus toxoid (TT) or diphtheria toxin (CRM197) are typically produced through fermentation using Clostridium tetani or Corynebacterium diphtheriae, respectively.
To produce the carrier protein, the gene encoding it, is inserted into a suitable host bacterium, such as Corynebacterium diphtheriae, using recombinant DNA technology. These genetically engineered bacteria are then cultured in bioreactors under controlled conditions to ensure high protein yield.
Harvest and clarification
Once sufficient bacterial growth is achieved, the culture medium needs to be harvested and clarified to remove other impurities. This is often performed through centrifugation or membrane-based techniques like tangential flow filtration (TFF). Tangential flow ultrafiltration, further clarifies the supernatant by removing remaining other larger particles.
Purification
The protein is then subjected to purification, typically involving ion exchange chromatography, affinity chromatography, or size-exclusion chromatography, to eliminate contaminants such as host cell proteins, endotoxins, etc.
After purification, aseptic filtration through a sterilizing grade 0.2µm filter is performed to ensure that the carrier protein is free from microbial contamination.
Carrier protein activation
The carrier protein undergoes chemical modification to introduce reactive groups that facilitate conjugation with polysaccharide. Following chemical modification, UF is used to remove any unreacted chemicals and byproducts. Finally, the purified and chemically modified carrier protein is formulated in a suitable buffer and sterile-filtered again.
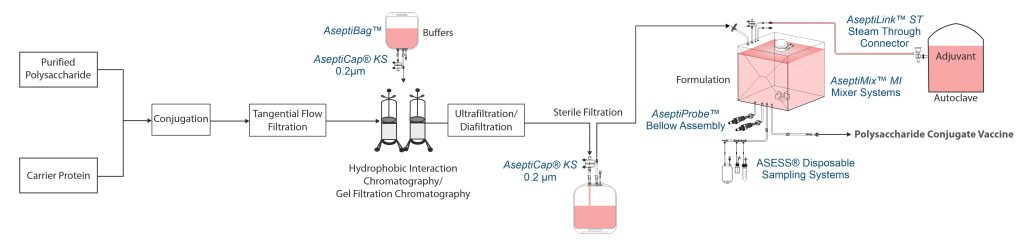
Conjugation
Activated polysaccharide is reacted with carrier protein, creating a covalent bond between them. For instance, PRP-ADH complex is conjugated to the carrier protein TT using an aldehyde method. Sodium metaperiodate activates PRP-ADH complex in specific buffer which is then mixed with activated TT and conjugation is completed within few days.
Purification of conjugate
Due to the increased molecular weight of the conjugate, a 300kDa membrane is used for concentration and partial removal of low-molecular weight impurities. Unreacted polysaccharide is separated using hydrophobic interaction chromatography (HIC), and sometimes gel chromatography filtration. After chromatography, the product is buffer-exchanged, then aseptically filtered through a sterilizing grade 0.2µm filter.
Formulation
Polysaccharide vaccines are either lyophilized or formulated with an adjuvant. Some tetravalent or pentavalent vaccines have multiple strains (D, T, P, Hib) and are formulated with adjuvants such as aluminium phosphate. As the final product cannot be filter sterilized, the antigen is sterilized separately and aseptically mixed with sterile adjuvant. For such vaccines (tetravalent/pentavalent) microbial cell culture and downstream processing of each strain are performed individually and blended in defined ratios before formulation