Virus-Like Particle (VLP) vaccines are a type of subunit vaccine that mimic the structure of actual viruses but do not contain any viral genetic material and are unable to multiply, making them non- infectious and safe.
Due to their high-density epitope display, ability to present multiple proteins to the immune systems, and size (approx 40nm) which appears to be ideal for dendritic cell uptake, VLPs elicit a high immunogenic response. Moreover, adjuvants are not expected to be required for VLPs to be highly immunogenic.
The virus-shell protein is expressed in cells during VLP manufacturing process. Numerous heterologous expression systems are capable of expressing VLPs.
There are numerous VLP-based vaccines in clinical trials and commercial distribution that are made in mammalian cell culture, plants (tobacco), baculovirus/ insect cell culture systems, and microbial fermentation (E.coli, yeast, etc).
Either the partially assembled protein is recovered from cell lysate and assembled into VLPs in vitro, or VLPs are assembled in vivo and then purified from cell lysate.
Types of VLP vaccines
| Disease | Disease causing microorganism |
Virus-Like Particle Vaccine |
| Cervical Cancer | Human Papillomavirus (HPV) | Gardasil, Cervarix |
| Malaria | Plasmodium (parasite) | Pfs25 VLP, Qβ-based VLPs |
| Hepatitis B | Hepatitis B virus (HBV) | Engerix – B, Euvax B |
Manufacturing of Virus-Like Particle (VLP) vaccines
The process begins with selecting an appropriate expression system such as bacterial, yeast, insect, mammalian or plant cells based on the complexity of the VLP and the need for post- translational modifications. Genes encoding viral structural proteins are cloned into expression vectors and introduced into the host cells, which then produce the proteins that self-assemble into VLPs. These particles are harvested from the cells or culture medium and purified using methods like centrifugation and chromatography to remove impurities. After purification, the VLPs undergo characterization to asses their size, shape, purity and stability.
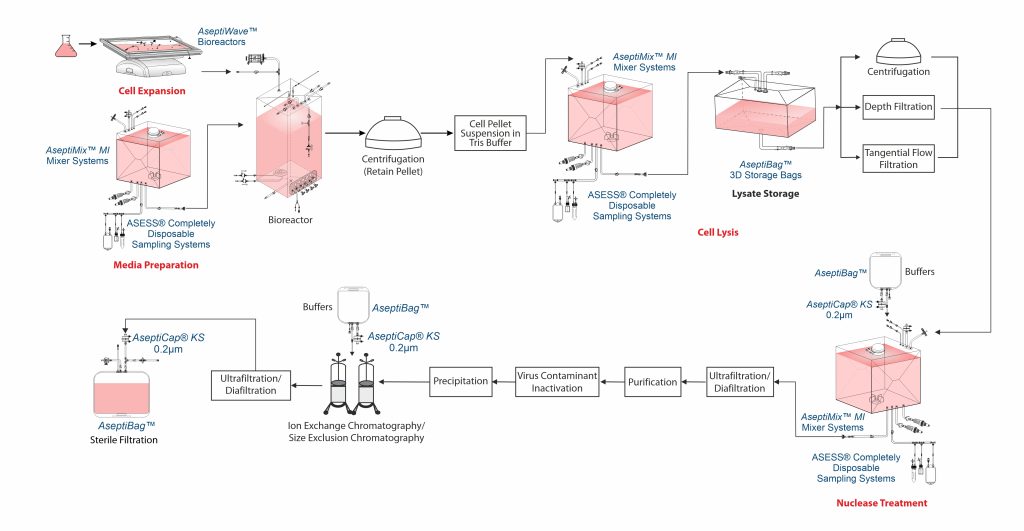
Cell cultivation
A functioning cell bank is typically grown using a series of shaking flask cultures, then moved to a bioreactor to grow until a specified culture volume is reached with an optimal cell density. Cells for VLP production are typically cultured in serum-free media, supplemented with fetal bovine serum (FBS), protein hydrolysates, lipid emulsions, and sometimes heparin to reduce cell aggregation. At 26°C – 28°C the cells are cultivated. Around 48 – 96 hours often infection, VLPs are collected.
However, yeast-based VLP production, particularly using S. cerevisiae, offers a cost-effective alternative to insect and bacterial systems and can be carried out in complete synthetic medium supplemented with glucose and yeast nitrogen base.
Typically, the culture is collected by centrifugation. After centrifugation, the supernatant is decanted, and the resulting cell pellet is stored at – 60°C to – 80°C.
Cell disruption
VLPs are purified by resuspending the cell pellet in Tris buffer, followed by microfluidization to obtain a cell lysate. Since most VLPs are expressed intracellularly, cell lysis is often necessary for their recovery. In certain cases, cell lysis is also employed to enhance yield. However, some influenza VLPs produced in insect cells have been reported to be secreted without the need for lysis.
Cell lysis releases host cell proteins (HCP) and host cell DNA (hcDNA), which must be removed, often through benzonase treatment. Common cell lysis methods include freeze-thaw, detergents, homogenization, sonication and most frequently, high-pressure lysis.
Chemically induced cell lysis can be performed in AseptiMix™ MI mixer bags with buffer bags and lysate storage integrated using AseptiBag™ 2D/ 3D, AseptiLink™ connectors, and Asess™ sampling systems.
Clarification
Centrifugation, depth filtration, or tangential flow filtration (TFF) are used to clarify cell or lysates, with microfiltration preferred for its scalability and robustness. High shear during lysis produces fine debris, which, along with the large size of VLPs, can complicate clarification. Debris removal is typically achieved using TFF, dead-end filtration (0.2µm) or centrifugation.
Nuclease treatment
Benzonase endonuclease is used during VLP purification to degrade residual nucleic acids, which are key contaminants with a regulatory limit of 10 ng/dose to ensure compliance with regulatory purity standards as per European Medicines Agency (EMA) and World Health Organization (WHO).
Benzonase endonuclease is typically used in batch incubation step, either before or after lysate clarification.
After incubation, benzonase endonuclease must be removed during downstream processing. If the enzyme is smaller than the VLP, TFF membrane retains VLPs while allowing the enzyme to pass. If the size difference is too small, ion-exchange chromatography can be used.
DNA digestion can be conducted in AseptiMix™ MI mixer bags with buffer bags, AseptiCap™ KS sterile filter for enzyme addition, and an Asess™ aseptic sampling system for QC testing.
Concentration and buffer exchange
Based on the VLP type, expression system and titer, an ultrafiltration/ diafiltration (UF/DF) step may be included to concentrate the product and perform buffer exchange in preparation for the next stage.
The retentate is typically diafiltered into Tris HCl, to prepare for ion-exchange chromatography.
Purification
VLPs are often purified by ultracentrifugation using CsCl, sucrose, or iodixanol gradients. However, CsCl should be avoided as it can lead to particle heterogenetity, introduce impurities, reduced functionality during storage and complicating Downstream Processing (DSP).
Virus contaminant inactivation and removal
Use of insect cell based processes may result in baculovirus contamination which, as per regulatory requirements has to be removed. Inactivation is performed prior to removal and this can be done using chemicals such as formalin and ß-propiolactone as an added safety.
Removal can be achieved through selective precipitation or chromatography methods. Chromatography steps for VLP purification are closely monitored to ensure separation from virus.
Polishing
Microbial systems such as E.coli, pose a challenge of endotoxins (lipopolysaccharides) and can be removed via ion exchange chromatography or membrane adsorption. However, due to VLP hydrophobicity the endotoxins interact with VLPs themselves. Solvents or detergents are used to liberate the bound endotoxins.
During the last polishing stages, weak ion exchange chromatography resins are frequently utilized. VLPs pass through the column during this process, and DNA and leftover baculovirus attach to the resin. The flow-through fractions are where the VLPs are gathered.
However, one or more Size Exclusion Chromatography (SEC) steps could be a useful substitute when VLP and some of the VLP derived impurities have similar electrostatic properties but there is a notable difference in their molecular size. Stabilizing chemicals are frequently used to reduce ionic interaction between SEC resins and VLPs.
At this stage, UFDF is a useful optional technique for eliminating low molecular weight contaminants. SEC & UFDF both work well for buffer exchange during final formulation.
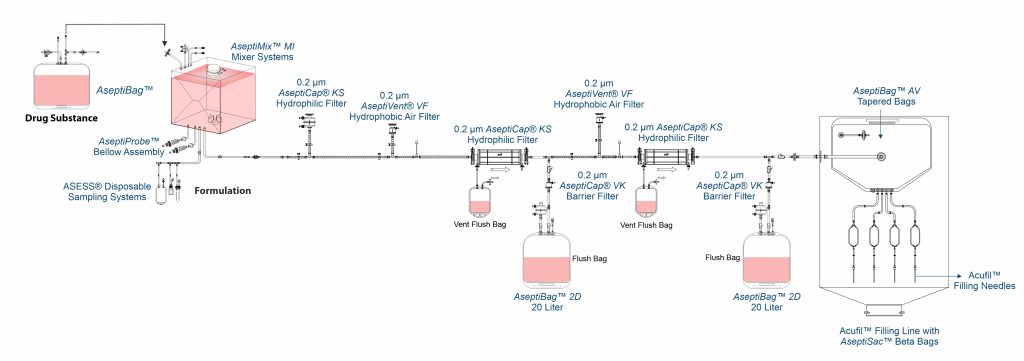
Formulation and Aseptic Filtration
Formulation of VLP based vaccines is done by aseptic filtration of different components using AseptiCap™ KS (PES membrane) or AseptiCap™ WS (PVDF membrane), which are then mixed aseptically using close disposable single use mixer systems such as AseptiMix™ MI.
MDI AcuFil™ disposable filling lines are used for filling of the final sterile formulation.