A virus is a pathogen that can only multiply within its host’s live cells. It cannot perform life functions like metabolism or reproduction on its own, so it is not regarded as a living organism. The size of a virus typically ranges from 10nm to 300nm, making viruses much smaller than bacteria and human cells.
A virus is made up of three structural parts
- The genetic material- DNA or RNA
- Protein coat (capsid)– composed of protein subunits known as capsomers which envelops the genetic material
- Outer lipid envelope– some viruses have outer lipid envelope that they obtain from the membrane of the host cell. Glycoproteins that aid in entry into host cells are frequently present in this membrane.
Viral vaccines are attenuated (weak) viruses which stimulate an immune response but are not virulent enough to cause the disease. Virus attenuation is done via passage-growing multiple times in unrelated or foreign hosts, such as tissue culture, embryonated eggs or live animals. It is likely that one of these passages will possess a mutation that enables to infect the host. However, compared to the virus in the original host, this mutant typically exhibits decreased pathogenicity. Certain contemporary vaccinations employ genetic engineering to specifically induce attenuation through gene substitution, deletion or selective mutation.
Advantages of attenuated vaccine
- Rapid onset of immunity
- Stimulate all arms of immune system (humoral, cellular, mucosal)
- Typically confer long-lasting protection
Limitations of attenuated vaccine
- Possibility of secondary mutations leading to reversion to virulence
- Risk of causing disease in immunocompromised individuals (e.g. people with AIDS)
- Require strict cold-chain conditions during transport and storage to keep the live virus viable
The production of attenuated virus vaccine is a multi-stage, intricate process. Each viral vaccine has a unique manufacturing process that is determined by host specificity, stability, shape, and size. Keeping the attenuated virus alive and preserving the viral vaccine's contagious capacity during formulation and downstream processing until it is given to healthy people is a major concern for vaccine manufacturing process owners.
Viral vaccine manufacturing process
The manufacturing of viral vaccine involves several key steps to ensure safety and efficacy. It begins with selecting and preparing the virus strain, which may be live-attenuated, inactivated, or genetically engineered. The virus is then grown in suitable host systems like animal cell lines, fertilized eggs, or yeast/insect cells. Once sufficient viral replication is achieved, the virus is harvested and purified using techniques like filtration and chromatography to remove impurities.
For inactivated vaccines, the virus is chemically killed while preserving its structure. The purified virus or viral components are then formulated with stabilizers and adjuvants, followed by sterile filling into vials.
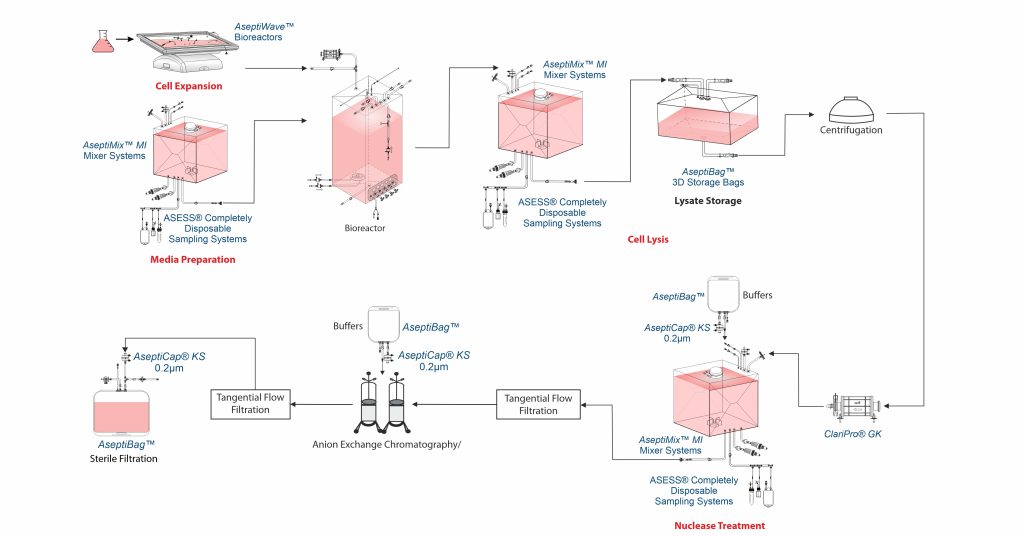
Virus culture and harvest
Viruses used in vaccine production are grown in cell cultures maintained in roller bottles, suspension systems or on microcarriers. Batch volume typically range from 500-700 L for roller bottles and 1000-2000 L for suspension cultures. Common cell lines include Vero, human diploid cells, MDCK and MRC-5, with vero cells being the most commonly used.
| Cell lines | Origin | Vaccines |
| MRC5 cells | Human lung derived diploid cells | Rabies virus, polio virus, hepatitis A virus, varicella virus, etc. |
| WI-38 cells | Human lung derived diploid cells | Varicella vaccine, rubella vaccine,etc. |
| MDCK cells (Madin-Darby canine kidney) | Kidney of a female Cocker Spaniel dog | Influenza vaccine |
| Vero cells | African green monkey kidney cells |
Polio vaccines (inactivated viral vaccine) Rotavirus vaccine and Small pox (live viral vaccine) |
In viral vaccine production, virus harvesting depends on the type of virus. Enveloped viruses (e.g. influenza, measles) are released from host cells through budding and are harvested while the cells are still viable. In contrast, non-enveloped viruses (e.g. poliovirus, rotavirus) require cell lysis to release the virus, which is done after sufficient intracellular replication. The method used ensures maximum virus yield while maintaining integrity for vaccine formulation.
Cell lysis can be performed in AseptiMix™ MI mixer bags with buffer bags and AseptiBag™ 2D/ 3D for lysate storage using AseptiLink™ sterile connectors and disconnectors.
Clarification
Clarification is the step where cells and cell debris are removed to collect the virus. This is often done using centrifugation, which separates particles based on their size and weight. However, filters are also commonly used for clarification. Filter choice depends on cell density, lysis extent and particle size.
ClariPro® GK helps in clarification of cell harvest supernatants to remove microfines, cell organelle, lipids and other colloidal particles. ClariPro® GK hydrophilic PES membrane large capsule filters are specially designed filters incorporating a microglassfiber upstream layer and a downstream PES membrane final layer.
Nuclease treatment
Virus harvest is followed by nuclease treatment (benzonase endonuclease at 30-34 °C for few hours) to breakdown host cell nucleic acids such as DNA or RNA, which are key contaminants with a regulatory limit of 10 ng/dose to ensure compliance with regulatory purity standards as per European Medicines Agency (EMA) and World Health Organization (WHO).
DNA or RNA digestion can be conducted in AseptiMix™ MI mixer bags with buffer bags, AseptiCap™ KS sterile filter for enzyme addition, and an Asess™ aseptic sampling system for QC testing.
Concentration and buffer exchange: TFF
Post nuclease treatment, the harvest undergoes TFF for concentration and diafiltration for buffer exchange preparing it for purification.
Purification
Benzonase treatment is often sufficient to purify attenuated vaccines such as mumps and measles. However, chromatography is needed for higher purity for vaccines like JEV. Common purification methods include anion exchange chromatography.
Post chromatography viruses are concentrated using TFF membranes.
Aseptic filtration
0.2µm sterilizing membrane filters are used to aseptically process the virus bulk.
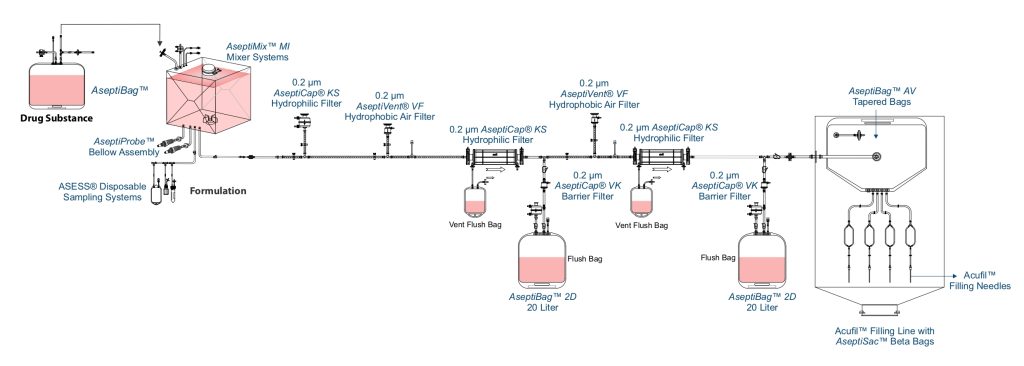
Formulation and Fill Finish
Most of attenuated viral vaccines are formulated using different strains to produce multivalent vaccines. Multivalent vaccine are specially designed to immunize against two or more strains of the same organism such as dengue vaccines (made up of 4 different strains- DENV-1, DENV-2, DENV-3 and DENV-4), polio vaccine (made up of 3 different strains- Type-1, Type-2 and Type-3), and rotavirus vaccine (made up of 5 different strains- G1, G2, G3, G4 AND G9).
In contrast, the MMR vaccine protects against three completely different viruses – measles, mumps, and rubella. It combines these distinct viruses into one shot, making it multivalent through the inclusion of multiple organisms.
Formulation of viral vaccines is done by aseptic filtration of different components using AseptiCap™ KS (PES membrane) or AseptiCap™ WS (PVDF membrane), which are then mixed aseptically using close disposable single use mixer systems such as AseptiMix™ MI.
MDI AcuFil™ disposable filling lines are used for filling of the final sterile formulation.