Live vector vaccines use weakened microbes to deliver antigens and trigger strong cell-mediated immunity, important for diseases like AIDS and malaria. These vaccines induce strong humoral and cellular immune responses, providing long-lasting immunity. They can be tailored to target specific cells based on viral tropism and may express multiple antigens, enabling protection against several diseases with a single vaccine. These vaccines are generally cost-effective and some are easy to transport. However, since they use attenuated viruses, there is a risk of reversion to virulence.
Vector vaccines use viral vectors to deliver genetic material to target cells. There are different types of viral vectors which can be used for vaccine manufacturing such as Adenovirus (70-90nm), Lentiviral vector (80-100nm), Canarypox (200-300nm), Alphavirus (70nm), Modified Vaccinia Virus Ankara (230nm), Attenuated yellow fever virus (50-90nm) etc.
Vectors like Canarypox and Modified Vaccinia Virus Ankara are too large to be filtered through sterilizing grade 0.2µm membrane filters, hence absolute aseptic manufacturing practices are required for these.
Adenovirus-based vaccines are the most commonly used ones and are engineered for safety and can express diverse antigens, triggering long-term immune responses. These are non-enveloped, double-stranded DNA viruses known for efficient cell transduction and high-yield production. Naturally, they can cause respiratory illness, but engineered adenoviral vectors are non-pathogenic and considered safe. Their manufacturing process is standardized and efficient.
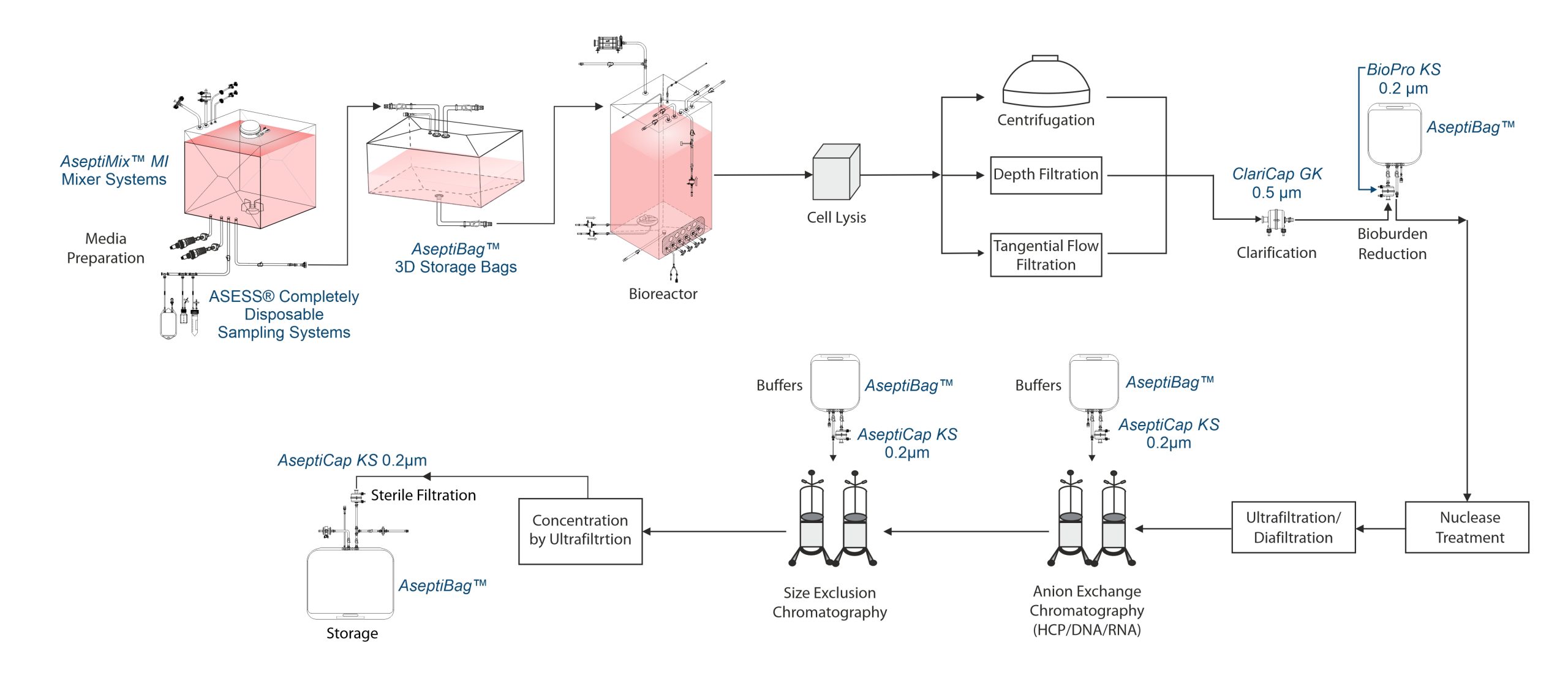
Viral vector manufacturing process
Adenovirus vectors are efficiently produced using engineered human cell lines (e.g. HEK 293) that support viral replication. These cells, with established safety profiles, are grown in serum-free suspension in stirred-tank bioreactor.
Harvest and clarification
Adenovirus is harvested by lysing cells, typically using Triton X-100. Clarification removes cell debris using filters (e.g. depth follow fiber) with options like TFF at low shear. Filter choice depends on cell density, lysis extent and particle size. Clarification is often followed by bioburden reduction (0.45µm filters ), reducing turbidity. Some processes also use centrifugation for initial clarification.
Nuclease treatment
Virus harvest is followed by nuclease treatment to breakdown host cell nucleic acids, which are key contaminants with a regulatory limit of 10 ng/dose.
Concentration and Diafiltration
Post nuclease treatment, the harvest undergoes TFF for concentration and diafiltration, retaining >99% of adenovirus. Some processes concentrate the vector before nuclease treatment. Buffer exchange via diafiltration prepares the product for chromatography. An overnight hold may be included, followed by filtration to reduce bioburden and protect chromatography columns.
Purification
Common purification methods include anion exchange (to remove HCP, DNA, RNA) and size exclusion chromatography (for trace contaminants).
Aseptic filtration
0.2µm sterilizing filters are used to aseptically process the final product.
