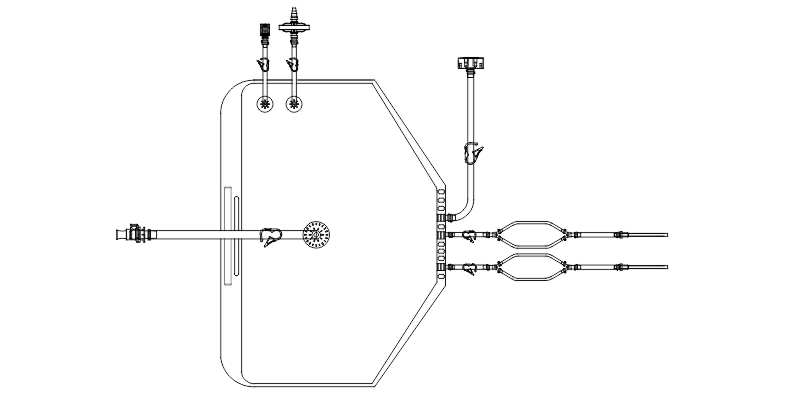
- Disposable filling lines for final fill and finish of high value drug products.
| Physical Properties of Film | |
| Storage Temperature | -20°C to 45°C |
| Materials of Construction | |
| Bag Film | AseptiFlex™ D film |
| Operational | |
| Sterilization | Gamma Sterilizable upto 50 kGy |
| Assurance | |
| 100% Integrity Tested | Each AseptiBag™ HV is tested for integrity to comply with validated Acceptable Integrity Test Specifications. |
| Bacterial Endotoxin | Aqueous extracts exhibit < 0.25 EU/ml as established by Limulus Amebocyte Lysate (LAL) Test as per USP <85> |
| Bioburden | Device bioburden is tested as per ISO 117 37-1 and assured to be <1000 cfu/bag. |
| Fiber Release | Passes microscopic test for fibers |
| Particle Release | Complies with USP <788> test for particulate matter in injections |
| Endotoxin Testing | Aqueous extracts exhibit <0.125 EU/ml as established by Limulus Amebocyte Lysate (LAL) Test as per USP <85> |
| Extractables | The Extractable study was performed as per Biophorum Best Practices Guide for Extractable Testing of Polymeric Single-Use Components used in Biopharmaceutical Manufacturing. |
| Sterility | The gamma sterilization process has been validated as per ISO 11137 to ensure a sterility assurance level (SAL) of 10-6 |
| Biosafety |
Passes the Biological Reactivity Tests, In Vivo for Class VI plastics as described in USP <88>.
Passes the Biological Reactivity Tests, In Vitro for Cytotoxicity as described in USP <87>.
|
MDI AseptiBag™ HV is only available as part of mdi single use systems
| File Type | Download |
|---|---|
| Datasheet | Download |
Have questions or need assistance?
Our team is here to help! Contact us today, and we’ll get back to you as soon as possible.