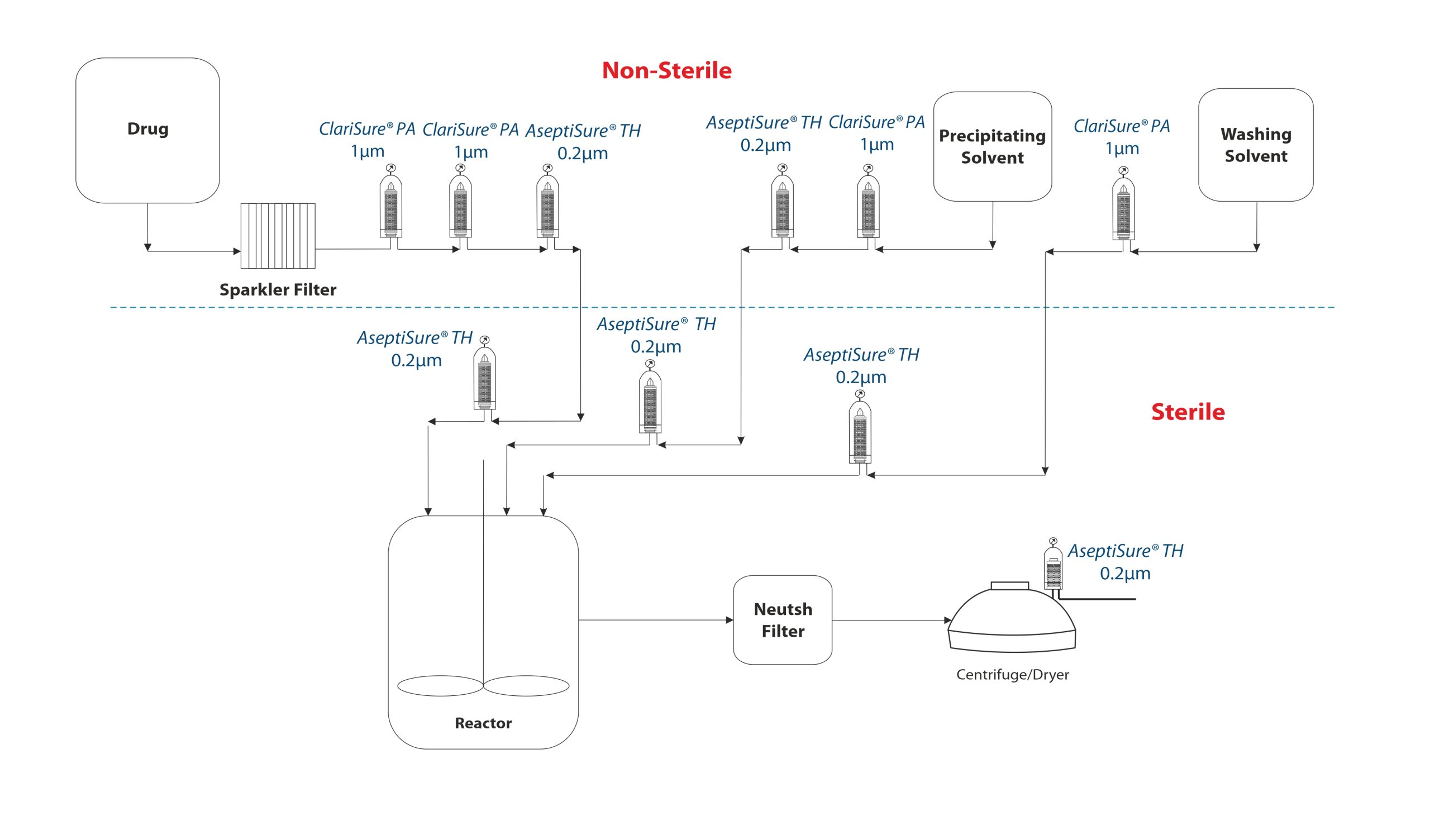
Active Pharmaceutical Ingredients (API’s), used as ingredients in sterile medicinal products, must be sterile unless the final dosage form is terminally sterilised, or produced by a process including a sterilizing filtration step. Sterile API’s intended for use in parenteral products must also comply with relevant specifications on pyrogens or bacterial endotoxins.
Therefore, since manufacture of sterile API’s must be strictly controlled in order to minimise the risk of contamination with micro-organisms, endotoxins and particles, sterile filtration is a critical process step.
MDI offers validated sterilization grade PTFE membrane cartridge filters with FEP encapsulated viton ‘O’ rings to ensure compatibility with a large variety of solvents that may be used in these processes.