Quality by Design in Single Use Systems
We are witnessing an industry wide shift in biopharmaceutical manufacturing processes from reusable stainless steel systems to single use disposable systems, for need of higher flexibility, faster turnaround and lower documentation and energy costs. However, since Single Use System (SUS) are customized multi-component polymeric assemblies, this has resulted in new challenges for the bioprocess owners with regards to leachables, biosafety, sterility, integrity and particulate matter.
The bioprocess owner as an end user, usually does not have the infrastructure or expertise to verify these single use assemblies for such regulatory and functional concerns and to ensure compliance. It has thus, become imperative for the SUS manufacturer/supplier to address these challenges through a well developed system that assures quality at every step to design, development and manufacture of these single use fluid management systems.
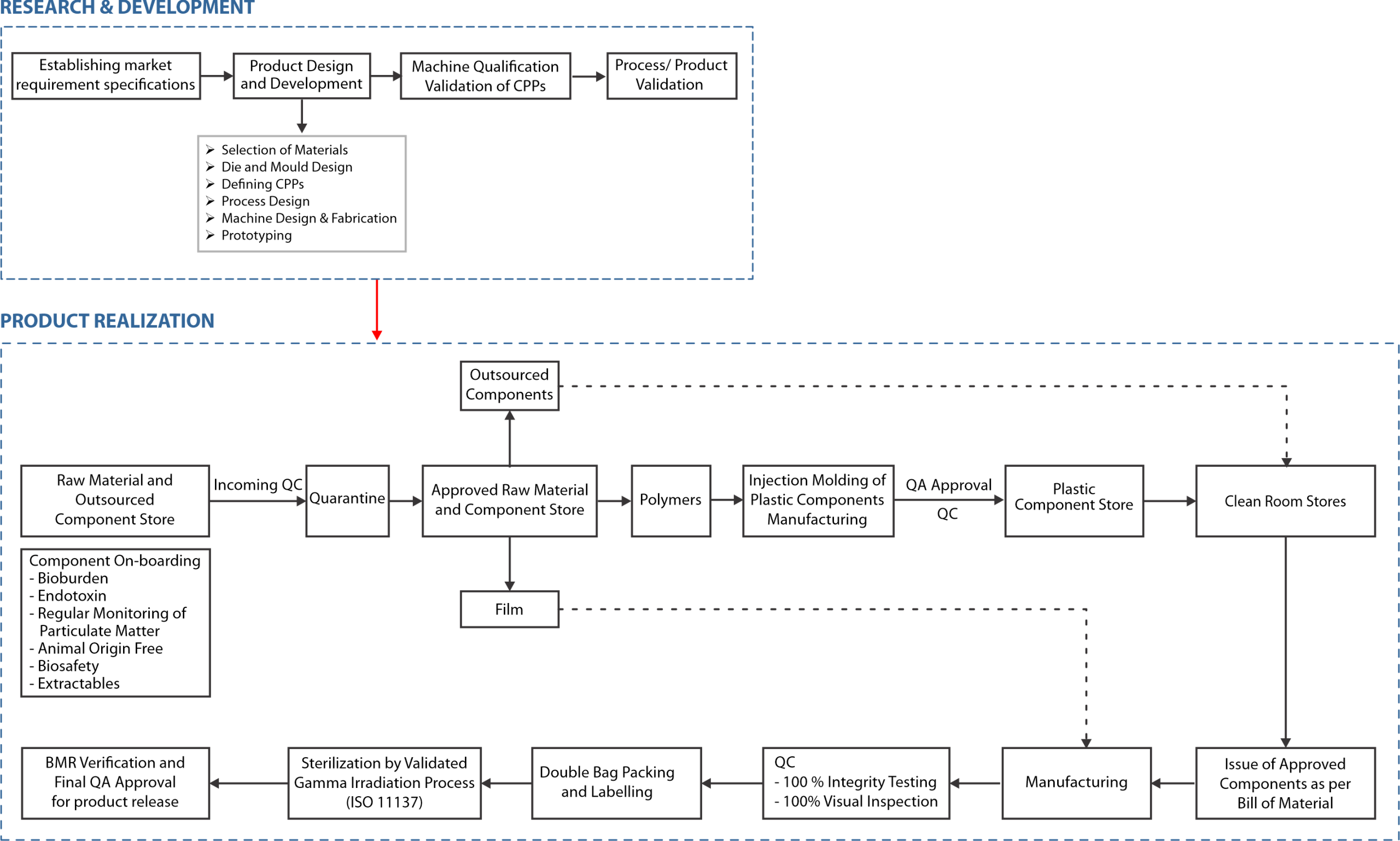
At mdi we corroborate this by incorporating the elements of Quality by Design at every step, starting with defining the Quality Target Product Profile (QTPP) by establishing market requirement specification (MRS). These specifications are based on a comparative analysis of competing products and customer feedback. This is followed by design and development of new product, including selection of materials, based on required Critical Material Attributes (CMA) and Critical Quality Attributes (CQA).
Once initial prototypes are tested, the productionization program which involves definition of various process steps involved in product realization along with Critical Process Parameters (CPPs) at each step, is initiated. This program culminates with machine qualification, validation of CPPs and process/product validation, and helps establish, not only a robust manufacturing process to deliver consistent quality but also to define test methodologies and specifications for in process and final product quality control.