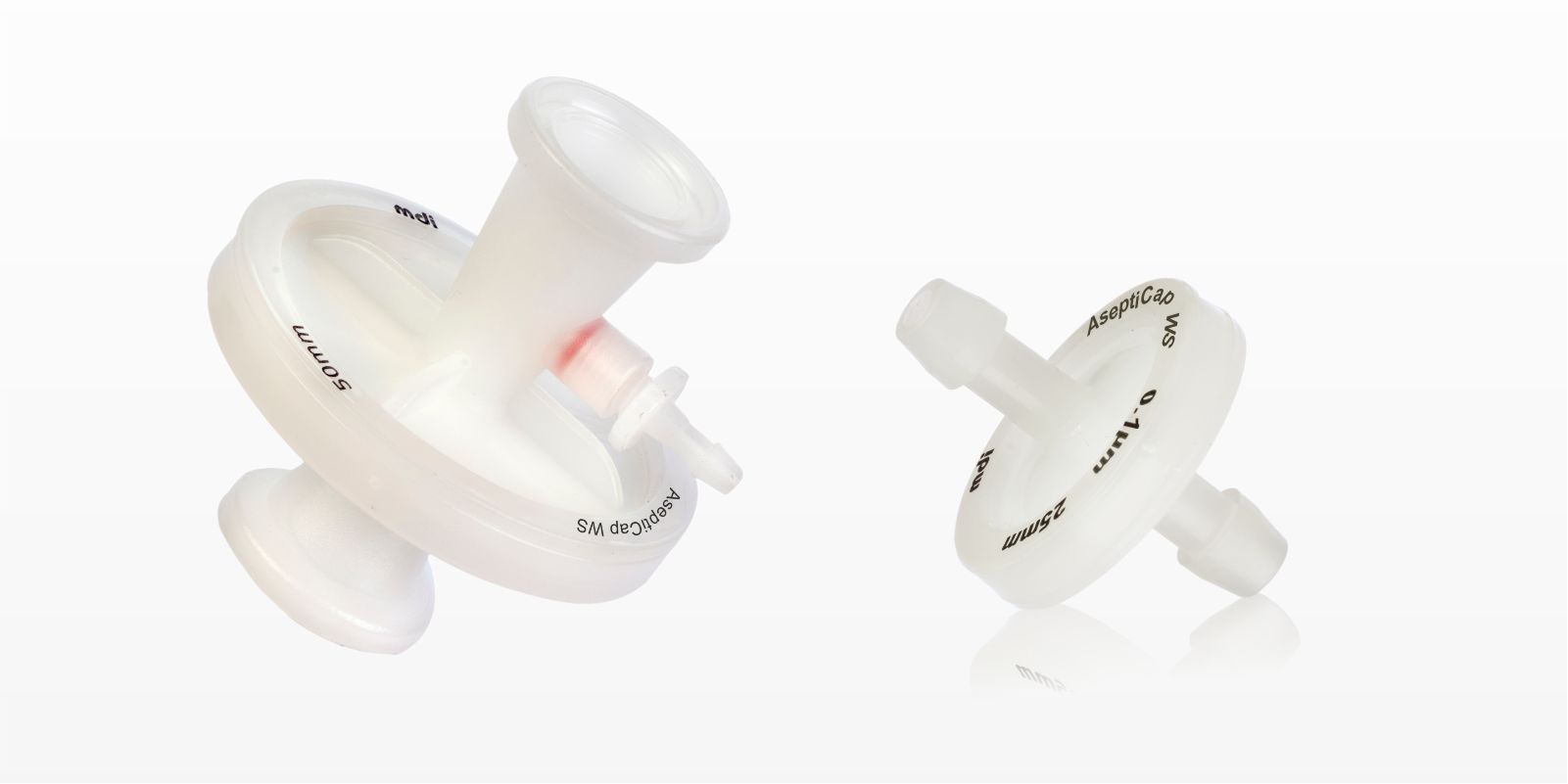
0.1µm AseptiCap® WL/WS Inline
MDI AseptiCap® WS are low protein binding hydrophilic PVDF gamma sterilizable membrane inline capsule filters, designed for sterile filtration of very small fluid volumes in formulation and process development labs.
These capsule filters are validated to meet compendia and regulatory requirements and are well characterized. They meet key process requirements such as absolute retention efficiency, extremely low extractables, high throughputs, wide chemical compatibility and other important characteristics.
- Absolute retention
- 100% integrity tested
- Low protein binding
- Very low hold-up volume in filters
- High flow rates
- Serial construction with prefilter for higher throughput with fouling streams
- Bioburden maintained below 1000 cfu/device
- Endotoxin level certified to be <0.25 EU/mL
- Wide range of end connections
- Products available for total scalability from a few ml to thousands of liters
- Total traceability through unique serial number for each filter
- Individual certificate of quality for each device
- Sterilizable by Autoclaving or EO
Sterile Filtration of:
- Cell culture media
- Growth regulators
- Small Volume Parenterals
| Construction | |||
| Membrane | 0.1 µm Hydrophilic PVDF | ||
| Prefilter Membrane | 0.2μm or 0.45μm Hydrophilic PVDF | ||
| Plastic parts | Polypropylene | ||
| Integrity Testing / Retention | |||
| Bubble Point | > 28 psi (1.96 Kg/cm²) with 50% IPA
> 70 psi (4.92 Kg/cm²) with Water |
||
| Microbial Retention | LRV>7 for Acholeplasma laidlawii (ATCC 23206) per cm² | ||
| Size | |||
| Size |
|
||
| Effective Filtration Area (Nominal) |
|
||
| Dimension (End to End) – Female Luer Lock Inlet/ Male Luer Slip |
|
||
| Dimension (End to End) – ¼” SHB |
|
||
| Dimension (End to End) – ¾” Sanitary Flange Inlet I/O |
|
||
| Operational Radius (with Vent/ Drain) |
|
||
| Operational | |||
| Max. Operating Temperature |
|
||
| Max. Differential Pressure |
|
||
| Sterilization By Gas | Sterilization by Ethylene Oxide | ||
| Sterilization By Autoclave | Autoclavable at 125°C for 30 minutes, 2 cycles and it cannot be in-line steam sterilized | ||
| Shelf Life | 2 years after EO sterilization | ||
| Assurance | |||
| Toxicity | Passes Bioreactivity test, In Vivo, as per USP <88> for Class VI plastics | ||
| Cytotoxicity | Passes Biological Reactivity Tests, In Vitro, USP <87> for cytotoxicity | ||
| Bacterial Endotoxin | Aqueous extracts exhibit < 0.25 EU/ml as established by Limulus Amebocyte Lysate (LAL) Test as per USP <85> | ||
| Bioburden | Bioburden level is < 1000 cfu/filter device as per ISO 11737-1 : 2018 | ||
| Non Fiber Releasing | Passes test as per USP and comply with USFDA 21 CFR Part 210.3(b)(6) for fiber release | ||
| TOC and Conductivity | Meets the WFI requirements of USP for TOC <643> and Conductivity <645> after a specified minimal flush | ||
| Indirect Food Additive | All Polypropylene components meet the FDA Indirect Food Additive requirements cited in 21 CFR 177.1520 | ||
| Extractables with WFI | Passes NVR test as per USP <661> | ||
| Oxidizable Substances | Passes test as per USP <1231> | ||
| Particle Shedding | Passes USP test for particulates in injectables. | ||
| Quality Management System | ISO-9001 Certified | ||
| USFDA | DMF No. 015554 | ||
0.1μm AseptiCap® WL/WS (25mm)
|
|
|
|
|
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
*Female Luer Lock is available as Inlet only
**Male Luer Slip is available as Outlet only
Example:
| IWS1 | 06 | 36 | M | N | X | X | 1 | 04 |
Example for Non Sterile: IWS10636MNRX104 Example for EO Sterile: IWS10636MNXX204
0.1μm AseptiCap® WL/WS (50mm)
|
|
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
*¼” Stepped Hose Barb and ¾” Sanitary Flange are available in filters with vent only
**¼” Single Step Hose Barb and Female luer lock is available in filters without vent only
Example:
| VWS1 | 10 | 36 | SS | X | X | 1 | 04 |
Example for Non Sterile: VWS11036SSXX104 Example for Gamma Sterile: VWS11036SSXX204
Have questions or need assistance?
Our team is here to help! Contact us today, and we’ll get back to you as soon as possible.