barrier filter, LVKX, LVKX5401QQXX301, LVKX5301QQRX101,LVKX54,LVKX53, AseptiCap VK-y, AseptiCap VK gamma, vk gamma
- Absolute retention
- 100% integrity tested
- Very low hold up volume in filters
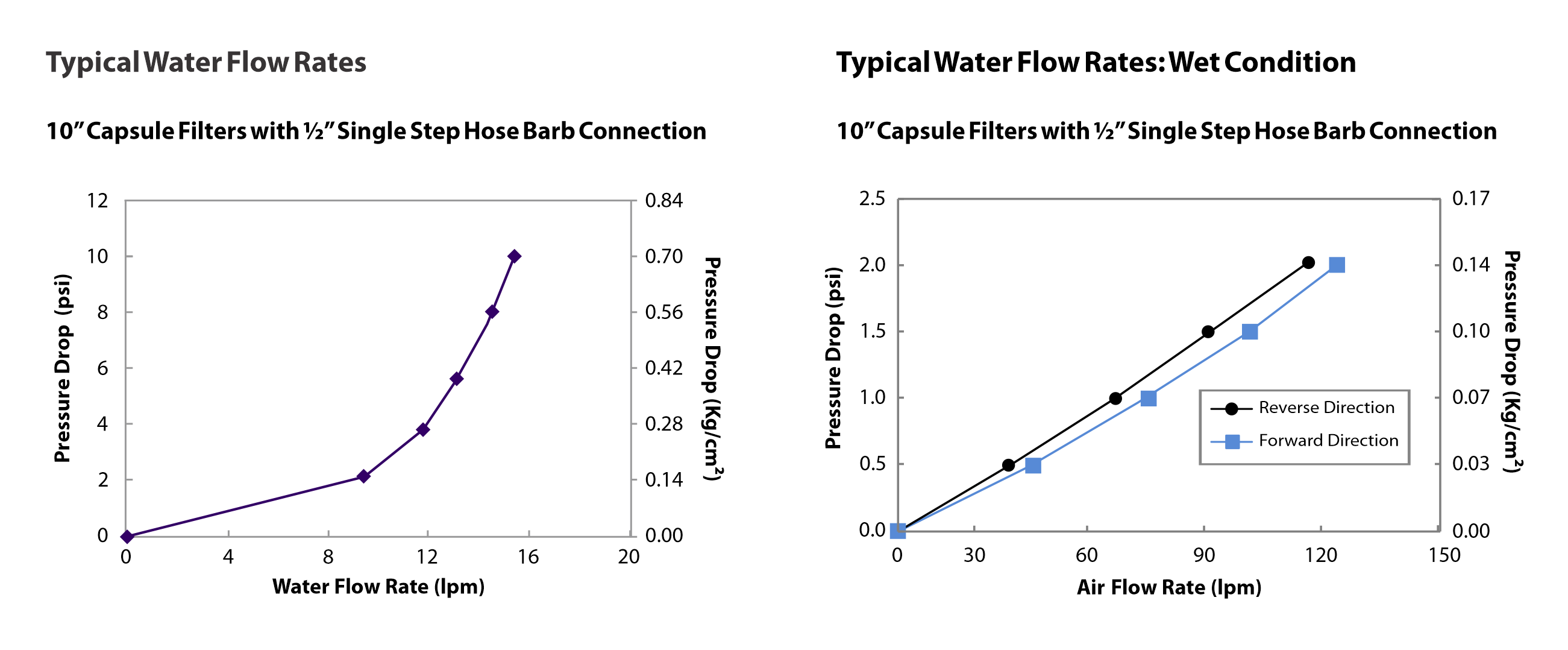
- High flow rates
- Bioburden maintained below 1000 cfu/device
- Endotoxin level certified to be <0.25 EU/ml
- Widest range of end connections
- Products available for total scalability
- Total traceability through unique serial number for each filter
- Sterilizable by Gamma irradiation
| Construction |
| Membrane |
Hydrophobic PVDF and Hydrophilic PES |
| Support Layers |
Polyester |
| Plastic parts |
Gamma Stable Polypropylene |
| Size |
| Size |
|
| Effective Filtration Area (Nominal) |
|
| Operational Radius (with Vent/ Drain) |
|
| Vent and Drain |
¼” Hose Barb with Silicone “O” ring |
| Integrity Testing / Retention |
| Bubble Point |
> 18 psi (1.26 Kg/cm²) with 50% IPA/water solution |
| Microbial Retention |
LRV >7 for Brevundimonas diminuta (ATCC 19146) per cm² |
| Operational |
| Max. Operating Temperature |
80 °C @ < 30 psi (2 Kg/cm²) |
| Max. Differential Pressure |
60 psi (4 Kg/cm²) @ 30 °C |
| Shelf Life |
2 years after gamma sterilization |
| Assurance |
| 100% Integrity Tested |
Each AseptiCap® VK-γ is tested for integrity to comply with
validated Acceptable Integrity Test Specifications. |
| Toxicity |
Passes Biological Reactivity tests, In Vivo, as per USP <88> for Class VI plastics |
| Cytotoxicity |
Passes Biological Reactivity tests, In Vitro, USP <87> for cytotoxicity |
| Bacterial Endotoxin |
Aqueous extracts exhibit < 0.25 EU/ml as established by Limulus Amebocyte Lysate (LAL) Test as per USP <85> |
| Non Fiber Releasing |
Passes test as per USP and comply with USFDA 21 CFR Part 210.3(b)(6) for fiber release |
| Sterilization by Gamma Irradiation |
Gamma Irradiatable up to 50 kGy.
These filters should not be autoclaved or in-line steam sterilized. |
| Indirect Food Additive |
Comply with USFDA 21 CFR Part 177.1520 |
| Bacterial Retention |
LRV> 7 for B. diminuta (ATCC 19146) per cm² of filter area as per ASTM F 838 |
| Oxidizable Substances |
Passes test as per USP <1231> |
| Quality Management System |
ISO-9001 Certified |
| USFDA |
DMF No. 015554 |
| Type | | AseptiCap® VK-γ | LVKX | | | Size | | Size | Code | | 5" | 53 | | 10" | 54 | | | Pore Size | | Pore Size | Code | | 0.2µm | 01 | | | I/O Connection | | Connection | Code | | Single Step ½" Hose Barb* | Q | | 1½" Sanitary Flange | E | | ¾" Sanitary Flange | S | | 3/8" Hose Barb* | I | | 1” Hose Barb | Z | | | Radiation Sterilizable | | Code | | Yes | R | | No* | X | | | | Sterility | | Code | | Non Sterile | 1 | | Gamma Sterile | 3 | | | Pack Size | | Pack Size | Code | | 1 | 01 | |
*Gamma irradiated filters can not be gamma sterilized again
Example for Non Sterile: LVKX5301QQRX101 Example for Gamma Sterile: LVKX5301QQXX301
- Allows unlimited water for injection (WFI) flushing of sterilizing grade product filter for easy wetting
- Allows fast drying of the filtration system necessary for processes involving oily solutions
- Acts as a sterile barrier against inadvertent ingress of environmental air