AseptiVent® TF 25mm, 37mm, 50mm
mdi AseptiVent® TF Disposable inline PTFE gas filters are convenient pre-fabricated devices used for sterilization of gases and as a bacterial air vent in various pharmaceutical and biopharmaceutical processes.
| Consistent and Reliable Quality: | AseptiVent® capsule filters are produced with ISO 9001 certified quality management systems. |
| Regulatory Compliance: | Meet all pharmacopeial and compendia requirements and are registered with USFDA DMF #015554 |
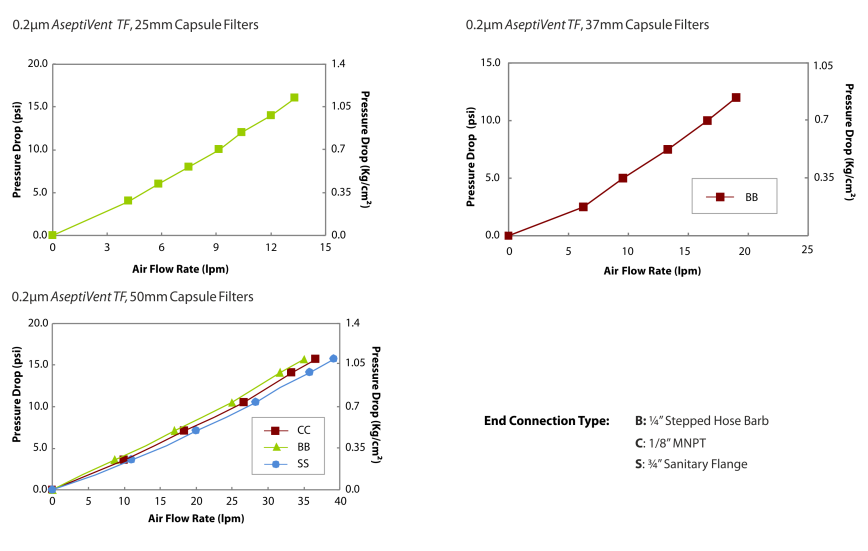
| Air Flow Rates: | AseptiVent® is produced using a high hydrophobicity PTFE membrane. This ensures good flow rates even with high moisture content in the inlet air. AseptiVent® capsule filters are designed to offer high air/gas flow rates at low differential pressures. |
- Sterile air for cell factories
- Venting of small bioreactors
| Construction | ||||
| Membrane | Hydrophobic PTFE | |||
| Pore size |
|
|||
| Support Layers | Polypropylene | |||
| Body and Core | Polypropylene | |||
| Size | ||||
| Size |
|
|||
| Effective Filtration Area (Nominal) |
|
|||
| Dimension (End to End) - Female Luer Lock Inlet/ Male Luer Slip |
|
|||
| Dimension (End to End) - ¾" Sanitary Flange |
|
|||
| Dimension (End to End) - ¼" SHB |
|
|||
| Dimension (End to End) ⅛" MNPT |
|
|||
| Operational Radius (with Vent/ Drain) |
|
|||
| Integrity Testing / Retention | ||||
| Bubble Point |
|
|||
| Microbial Retention |
|
|||
| Operational | ||||
| Max. Operating Temperature | 60°C | |||
| Max. Differential Pressure | 3Kg/cm² (42 psi) @ 30 °C | |||
| Burst Pressure |
|
|||
| Sterilization By Gas | Sterilizable by Ethylene Oxide | |||
| Sterilization By Autoclave | Autoclavable at 125°C for 30minutes, 30 cycles and it cannot be in-line steam sterilized | |||
| Shelf Life | 3 years after EO sterilization | |||
| Assurance | ||||
| Toxicity | Passes Bioreactivity test, In Vivo, as per USP <88> for Class VI plastics | |||
| Cytotoxicity | Passes Biological Reactivity Tests, In vitro, USP <87> for cytotoxicity | |||
| Bacterial Endotoxin | Aqueous extracts exhibit < 0.5 EU/ml as established by Limulus Amebocyte Lysate (LAL) Test | |||
| Bioburden | Bioburden level is < 1000 cfu/filter device as per ISO 11737-1 : 2018 | |||
| Microbial Retention | Validated as per ASTM F 838 | |||
| Non Fiber Releasing | Passes test as per USP and comply with USFDA 21 CFR Part 210.3(b)(6) for fiber release | |||
| Indirect Food Additive | All Polypropylene components meet the FDA Indirect Food Additive requirements cited in 21 CFR 177.1520 | |||
| Good Manufacturing Practice | These products are manufactured in a facility which adheres to Good Manufacturing Practices | |||
| Oxidizable Substances | Within limits as specified in USP <1231> | |||
| Particle Shedding | Passes USP test for particulates in injections | |||
| Quality Management System | ISO-9001 Certified | |||
| USFDA | DMF No. 015554 | |||
AseptiVent®- TF (25mm)
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||
Example :
| ITFX | 06 | 01 | MN | XX | 1 | 04 |
AseptiVent®- TF (37mm, 50mm)
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
* Note: AseptiVent® TF- 37 mm is available with BB connection only
** 37mm filter are available in pack of 20 only
Example :
| ITFX | 08 | 01 | BB | XX | 1 | 09 |