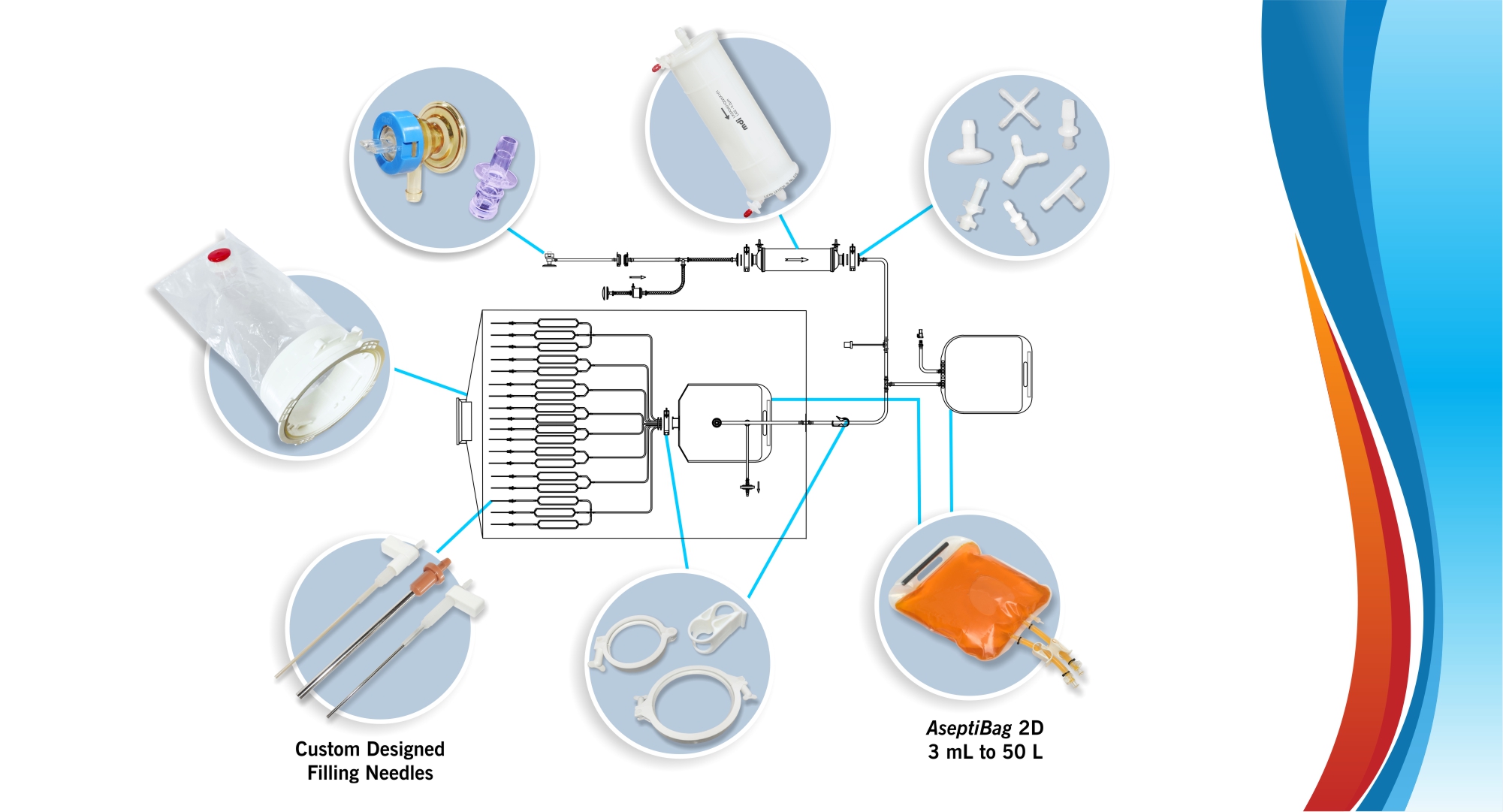
The COVID-19 pandemic, due to the enormous demand/supply gap for vaccines, exposed the supply chain problems in the biopharmaceutical industry. The entire industry was gasping for critical single use fluid management systems (SUS) and lead times extended up to almost a year (52 weeks). This was partly because of poor idle capacities with SUS manufacturers and over dependence for single use components on suppliers, who in turn had limited capacities to deliver large quantities within the required time.
MDI, a highly vertically integrated company, with the ability to develop and manufacture membranes, films, and plastic components from basic polymers and to deliver sterilizing filters and single use systems, met this seemingly insurmountable challenge by:
- Adding 100,000 sq ft of clean room area and rapidly expanding its productions by almost 6 times.
- Reducing lead times to 8-10 weeks through development and manufacture of a wide range of single use components to overcome the supply issues from component suppliers.
Further, MDI’s abilities to innovate and rapidly develop prototypes helped not only reduce delivery times but also improve process efficiencies by reducing system complexities.
Biopharmaceutical companies rely on MDI single use systems for critical applications in their processess. The wide range of capabilities and high level of quality has resulted in a substantial growth in business over the last one year.
MDI today is a reliable supplier for single use systems and is serving biopharmaceutical companies world-wide as they look to diversify their supply chain and lower risks.