Sterile drug products produced by aseptic processing involve filtration through sterilizing grade filters. It is critical for the drug manufacturer to ensure that the sterilizing filter is integral during the entire filtration process, resulting in a sterile downstream. Regulatory bodies worldwide have therefore made it mandatory to test filter integrity post use and mitigate risk to patient safety.
However, studies* have shown that in some drug products, filter clogging has an impact on post use integrity test (Bubble Point) values, thereby highlighting the possibility of a non integral filter passing post use integrity test due to masking of pre-use flaws.
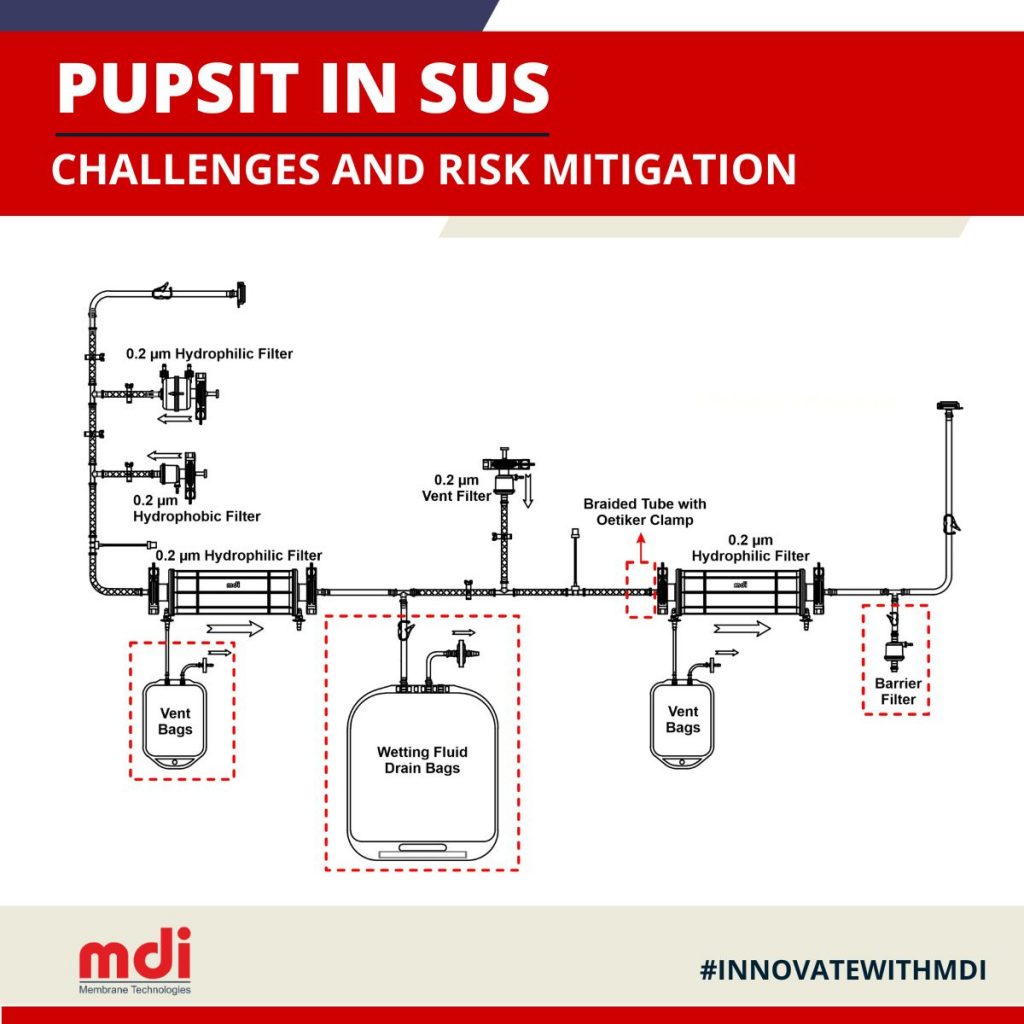
All this has resulted in a heightened focus on Pre-Use Post-Sterilization Integrity Testing(PUPSIT) and most of the regulatory bodies have made it mandatory. However, with the ever increasing use of single use systems (SUS) in the manufacture of sterile drug products, PUPSIT has become even more challenging.
Continue reading “PUPSIT in Single Use Systems: Challenges and Risk Mitigation”

