Sterile drug products produced by aseptic processing involve filtration through sterilizing grade filters. It is critical for the drug manufacturer to ensure that the sterilizing filter is integral during the entire filtration process, resulting in a sterile downstream. Regulatory bodies worldwide have therefore made it mandatory to test filter integrity post use and mitigate risk to patient safety.
However, studies* have shown that in some drug products, filter clogging has an impact on post use integrity test (Bubble Point) values, thereby highlighting the possibility of a non integral filter passing post use integrity test due to masking of pre-use flaws.
All this has resulted in a heightened focus on Pre-Use Post-Sterilization Integrity Testing(PUPSIT) and most of the regulatory bodies have made it mandatory. However, with the ever increasing use of single use systems (SUS) in the manufacture of sterile drug products, PUPSIT has become even more challenging.
The multiple risk factors associated with PUPSIT of single use disposable sterilizing filtration systems are:
- Inadequate filter wetting resulting in false integrity failure
- Downstream contamination during wetting fluid drainage and test gas venting
- Handling of large flush volumes required for wetting of large sterilizing filters
- Wetting fluid incompatibility with the drug product
- Leakages due to high pressure (> 4 bar) during integrity testing
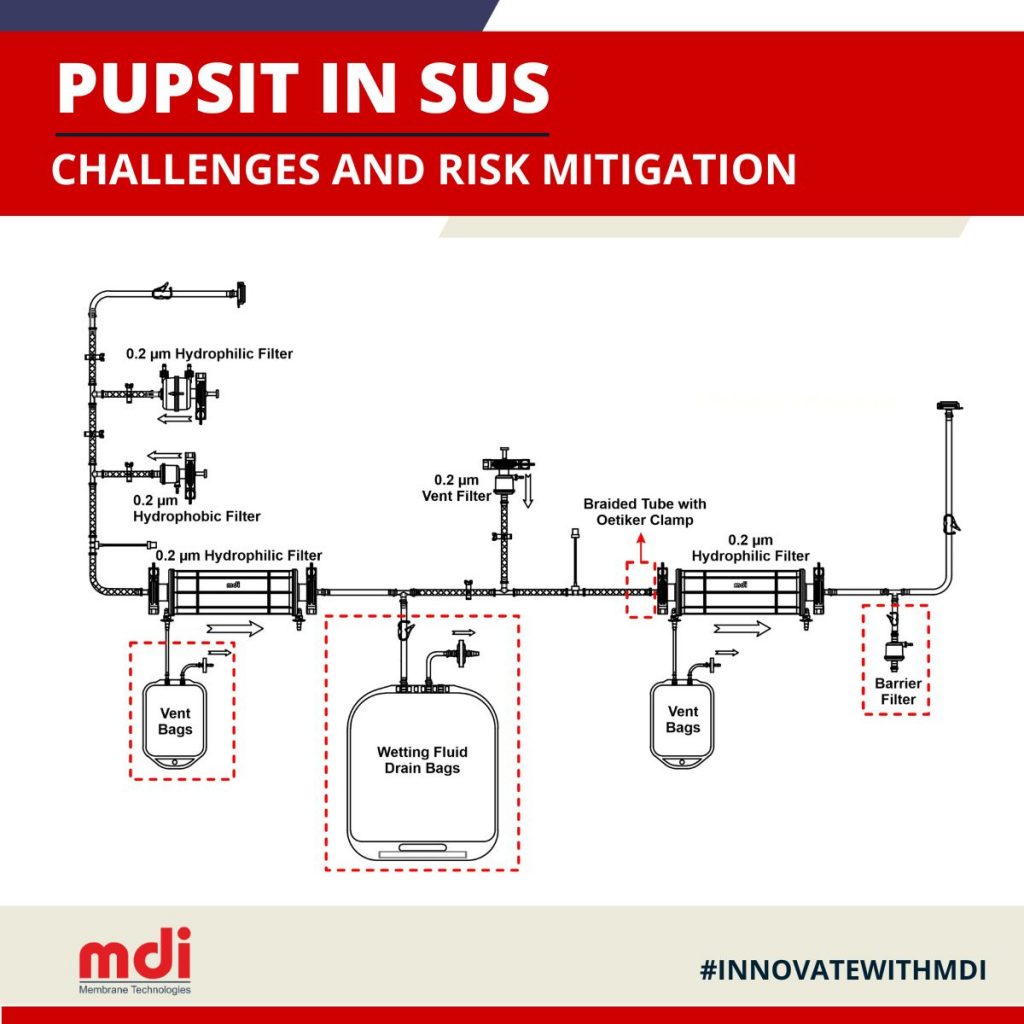
– Recommended SUS for Risk Mitigation
- Adequate filter wetting
Use of vented bags on filter vents to ensure removal of entrapped air and drain bags for collection of wetting fluid while preventing any spillage of wetting fluid into the sterile environment
- Handling of large flush volumes during filter wetting
Although vented flush bags downstream of sterilizing filters ensure wetting fluid drainage and test gas venting without exposure to the environment, large sterilizing filters may require large volumes of wetting fluid and handling large capacity flush bags may pose a risk to operator safety. Hydrophobic/Hydrophilic barrier filters mitigate such risks by easy handling of large flush volumes without microbial ingress from the environment.
- PUPSIT with drug product as wetting fluid
Blow drying incompatible wetting fluids requires very high pressures and is not practically feasible in single use systems, thereby necessitating PUPSIT with the drug product as wetting fluid. However, this can be prohibitively expensive in case of high value drug products. Scaled bags can help minimize such costs.

Mitigating risk of leakages
Use of braided tubes and high pressure non-braided tubes along with Oetiker clamps ensure secure connections and prevent leakages.
* https://journal.pda.org/content/early/2019/11/15/pdajpst.2018.009449

