We are witnessing an industry-wide shift in biopharmaceutical manufacturing processes from reusable stainless steel systems to single use disposable systems, due to the need for higher flexibility, faster turnaround time and lower documentation and energy costs.
Since Single Use Systems (SUS) are customized multi-component polymeric assemblies, this shift has resulted in new challenges for the bioprocess owners with regards to leachables, biosafety, sterility, integrity and particulate matter.
The bioprocess owner as an end user, usually does not have the infrastructure or expertise to verify these single use assemblies for such regulatory and functional concerns and to ensure compliance. It has thus become imperative for the SUS manufacturer/supplier to address these challenges through a well developed system that assures quality at every step of design, development and manufacture of these single use fluid management systems.
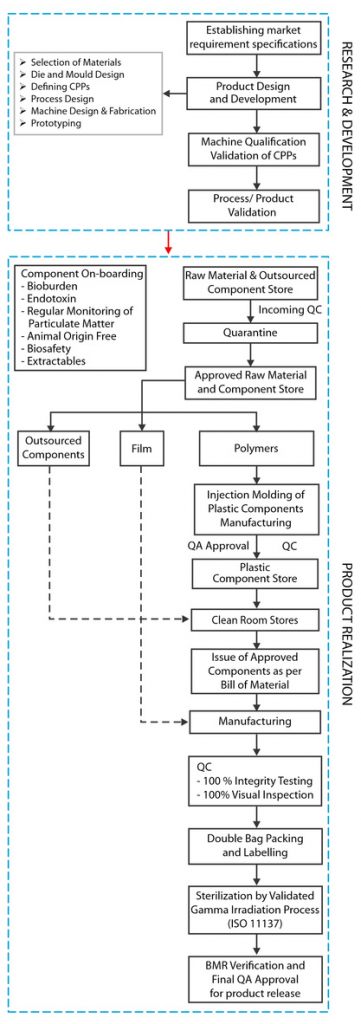
At mdi we corroborate this by incorporating the elements of Quality by Design at every step, starting with defining the Quality Target Product Profile (QTPP) by establishing market requirement specification (MRS).
These specifications are based on a comparative analysis of competing products and customer feedback. This is followed by design and development of new products, including selection of materials, based on required Critical Material Attributes (CMA) and Critical Quality Attributes (CQA).
Once initial prototypes are tested, the productionization program which involves definition of various process steps involved in product realization along with Critical Process Parameters (CPPs) at each step, is initiated. This program culminates with machine qualification, validation of CPPs and process/product validation, and helps establish, not only a robust manufacturing process to deliver consistent quality but also to define test methodologies and specifications for in process and final product quality control.
SUS at mdi are manufactured in GMP compliant ISO class 7 clean room facilities by trained and qualified personnel. The manufacturing environment as well as products manufactured, are regularly monitored for bioburden, particulate matter and endotoxins. Each single use assembly lot is subjected to 100% integrity testing and 100% visual inspection and the final product is sterilized by a validated gamma irradiation process.
Finished products are released into the market after Batch Manufacturing Records verification and approval by Quality Assurance.

