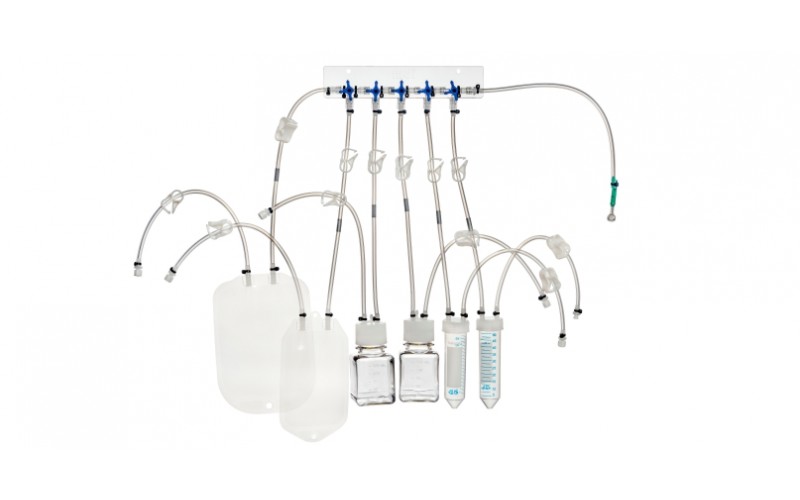
MDI ASESS Aseptic Sampling System is designed for sampling biopharmaceuticals and other process fluids for analytical purposes such as:
- Bio-burden
- Endotoxin
- Sterility Testing
- pH Testing
- Chemical Analysis
The Aseptic Closed Sampling Systems are used to achieve multiple sampling from sterile containers such as process vessels and bioreactors while complying with regulatory guidelines
Unique Features:
- Closed system: Prevents extraneous contamination
- Artifacts free clean system
- Personnel safety from biohazardous and cytotoxic fluids
- Cost advantage through customization
- Easy and aseptic sampling for different tests
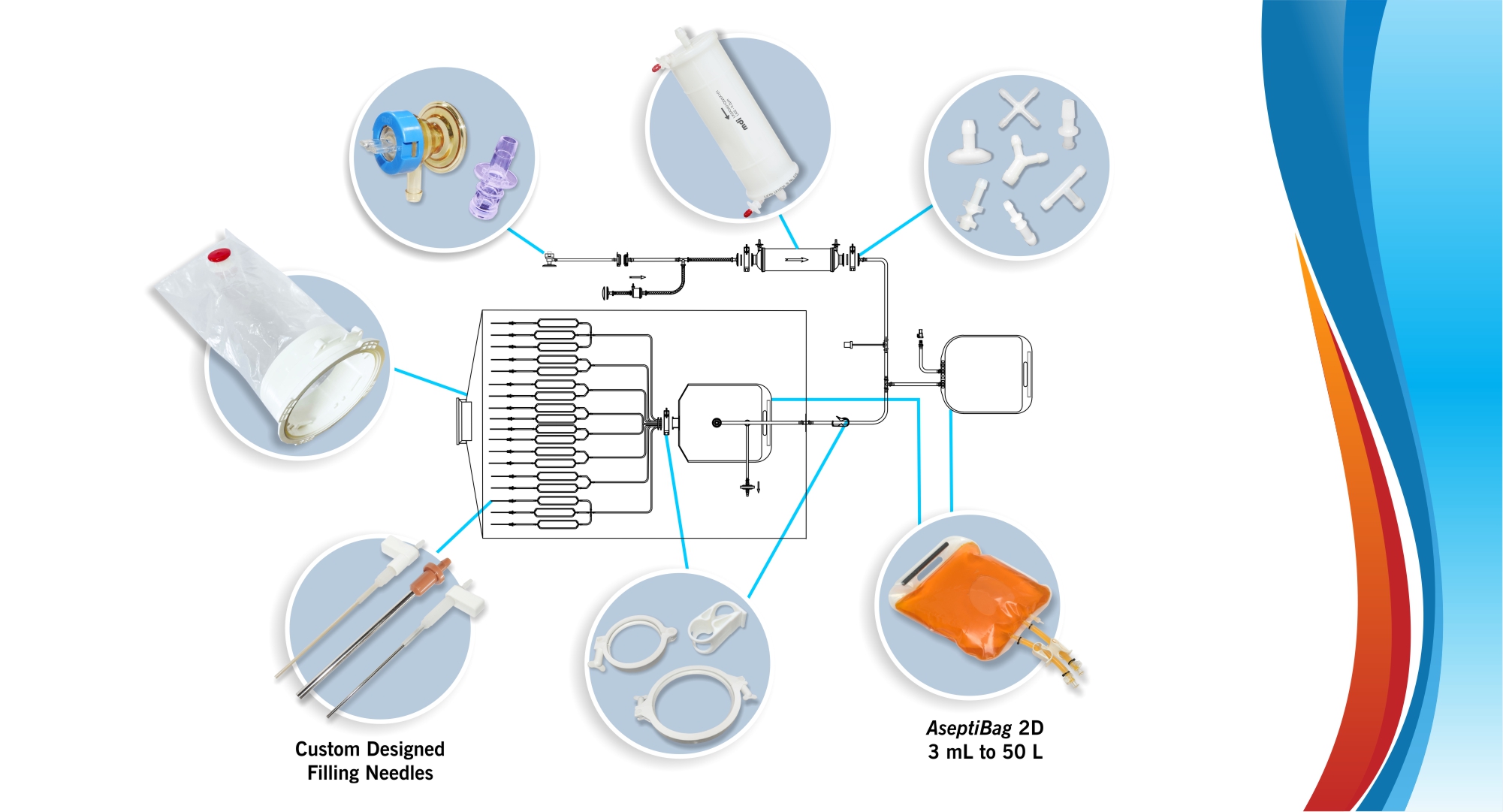
Asess sampling port is available with the following needles:


Customization:
ASESS Aseptic Sampling Systems can be customized to suit user requirements regarding the number and type of sampling containers such as bags, bottles, and centrifugal tubes.
A technical feasibility of the required design is established based on available components and an initial drawing is proposed. Product prototyping and final approval leads to customized system realization.