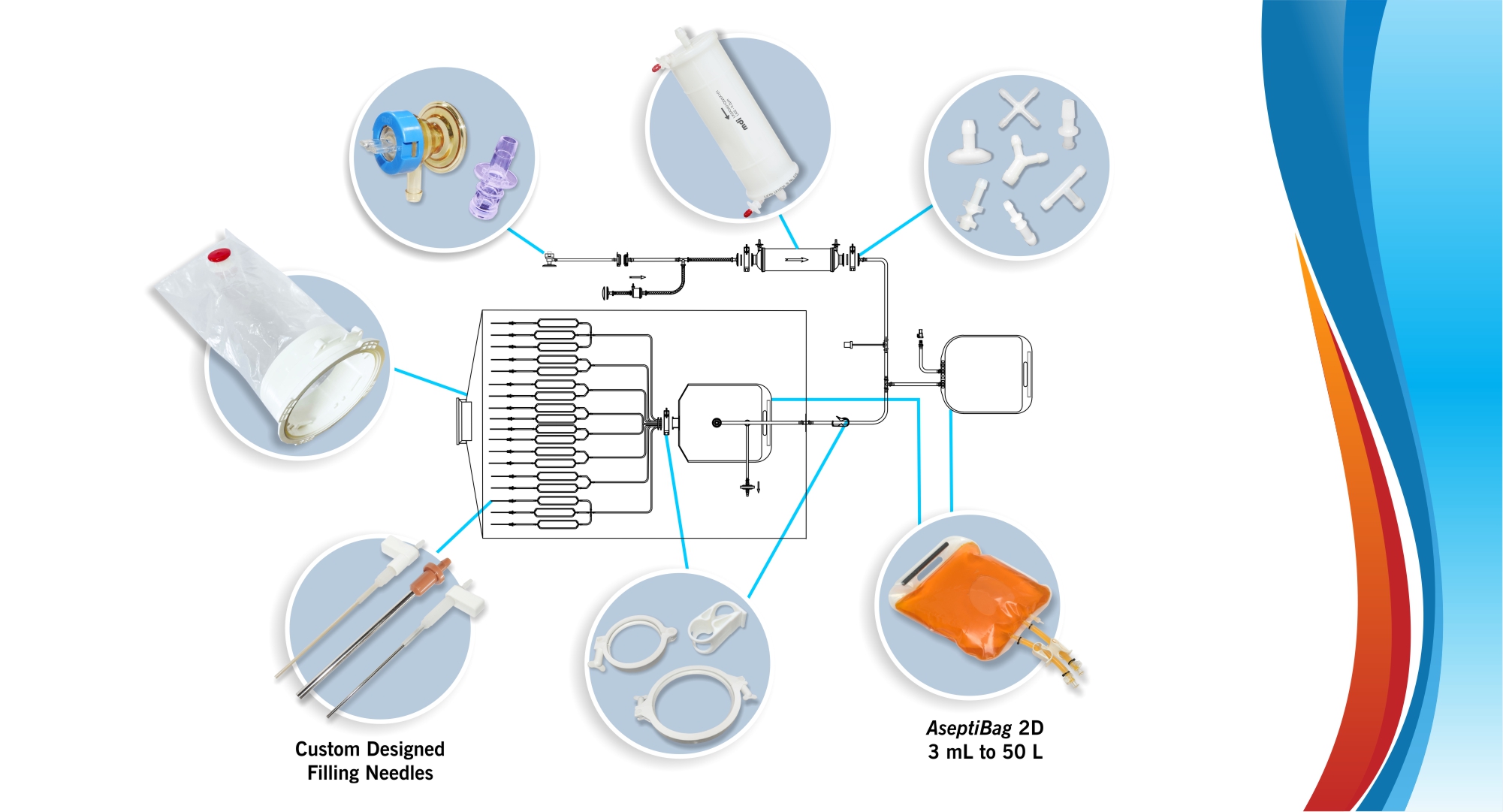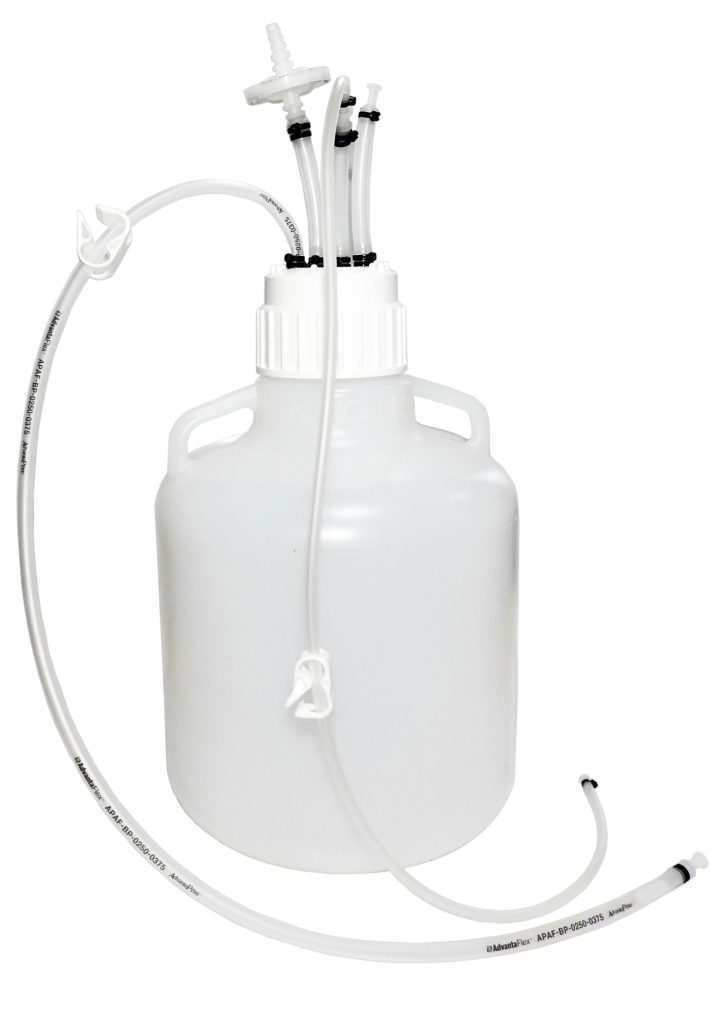
MDI AseptiMix VC are gamma sterilized vented mixer assemblies, suitable for mixing, safe transfer, and storage of biopharmaceutical products and reagents.
The assembly does not require any additional hardware and can directly be placed on a magnetic mixer for mixing with a stir bar placed inside.
The PVDF stir bar inside the mixer assembly has wide chemical compatibility and it ensures the proper mixing of solution without any particle shedding.
These assemblies are fitted with a self-supporting lightweight, sterilizing grade 0.2μm PVDF vent filter to prevent ingress of microorganisms during filling and removal of high-value products.
These assemblies are available with capacities of 10 liters and 20 liters.
Applications:
- Mixing, storage, and transfer of cell culture media and buffers
Customization:
MDI Vented Bottle assemblies can be customized to suit user requirements in terms of tubing, fittings, end connections, and connectors.