Mixing is a critical process requirement at multiple steps in Biomanufacturing. Various processes that involves mixing operation range from liquid-liquid mixing and solid-liquid mixing of different fluids such as media, growth regulators, harvests, post centrifuge supernatants, buffers, process intermediates and formulations for a biopharmaceutical process. Conventionally, fixed stainless steel systems were used for these processes consisting of glass bottles, large carboys or stainless steel vessels. These traditional systems faced multiple challenges in terms of extraneous contamination, cleaning & validation requirements and flexibility. Several life cycle assessment reports on use of single use systems and conventional fixed systems demonstrated the high environmental impact of fixed systems which are driven by huge water and energy consumption required for cleaning and sterilization processes during the use phase of product life cycles compared to gamma sterilized single use systems which eliminate the need for cleaning and validation and provide process flexibility.
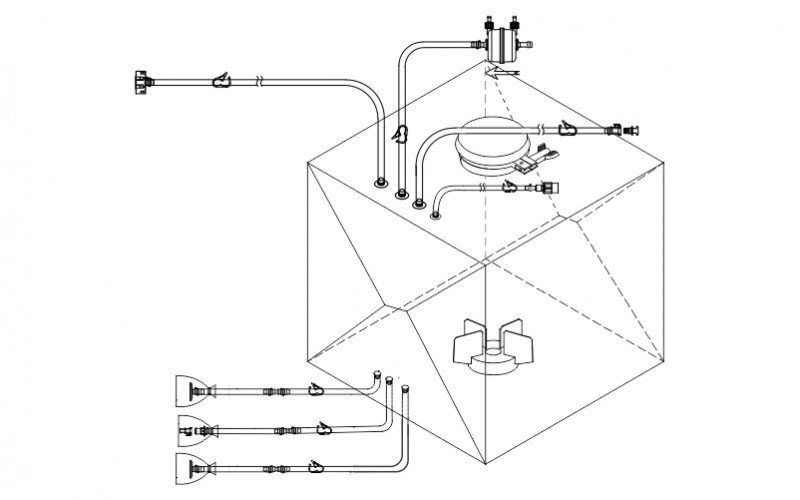

The above considerations and challenges have led biopharmaceutical manufacturing process owners to adopt disposable single use systems and make a shift from stainless steel systems as it allows quick , economical and scalable production and can be adapted to nearly any process.
MDI AseptiMix MI 3D mixer bags provide validated and reliable mixing platform for biopharmaceutical process such as mixing of media, process intermediates, sterile buffers with wide ranging pH, and formulations. mdi AseptiMix MI mixer bags come with a magnetic impeller and are available for volumes up to 1000 liters. These are also available with 4” and 8” sanitary flange powder port for powder-to-liquid mixing.
Mixing operation of AseptiMix MI single use mixer bags is carried with magnetic impeller which is coupled with pre-fitted magnetic motor drive unit on mdi BioMixer MI mixer systems through magnetic forces. There are no dynamic seals or shaft penetration inside the mixer bag. By switching ON the motor through BioMixer MI control panel, rotation of the impeller is induced inside the AseptiMix MI mixer bag with the magnetic motor drive, offering uniform and fast mixing with very low extractable profile for low ‘Product risk’.
Customization
MDI works closely with biopharmaceutical manufacturing process owners to understand their requirements and offer customized mixer systems to fit their existing hardware from different suppliers. Custom designed mixer systems are available with wide range of pre-qualified components such as membrane capsule filters, connectors, tubing, fittings etc.
Validation
MDI AseptiMix MI mixer are deeply characterized and validated for mixing efficiency, bacterial endotoxins, sterility, biosafety and extractables to minimize ‘product risk’ and maximize regulatory compliance enabling a strategic and unencumbered mixing workflow, presenting the most efficient, economical, scalable, readily integrated, operational and sustainable features.

