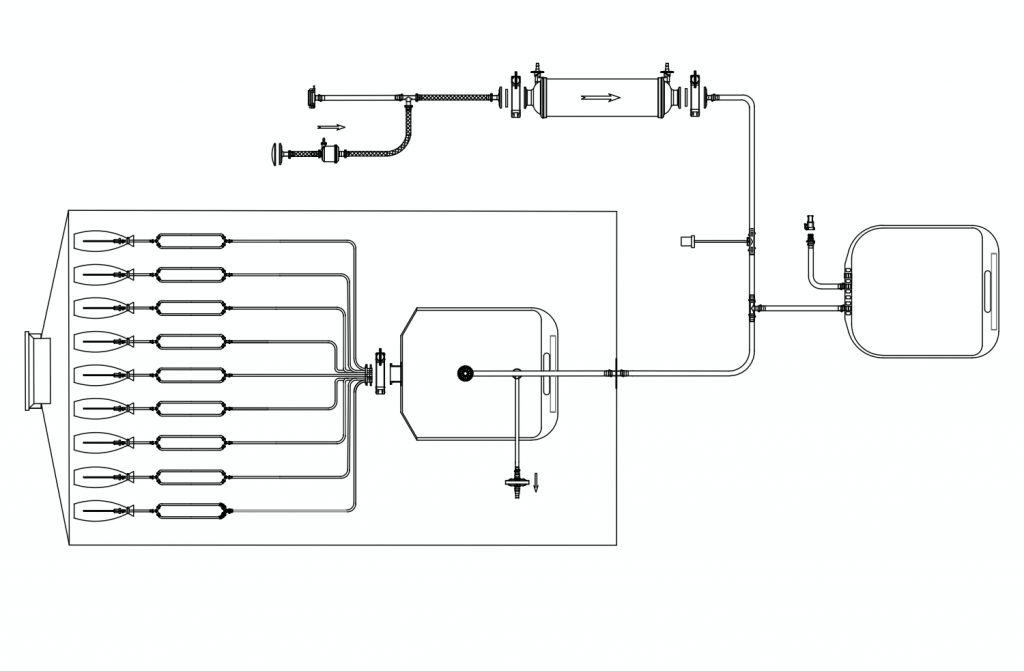
Scientists at Biopharmaceutical R&Ds and process owners involved in the manufacture of high value products such as radiopharmaceuticals, are looking for ready to use customized, disposable mixing and transfer solutions that are easy to use and have a small footprint given the limited availability of LAF(Laminar Air Flow) Space for aseptic operations.
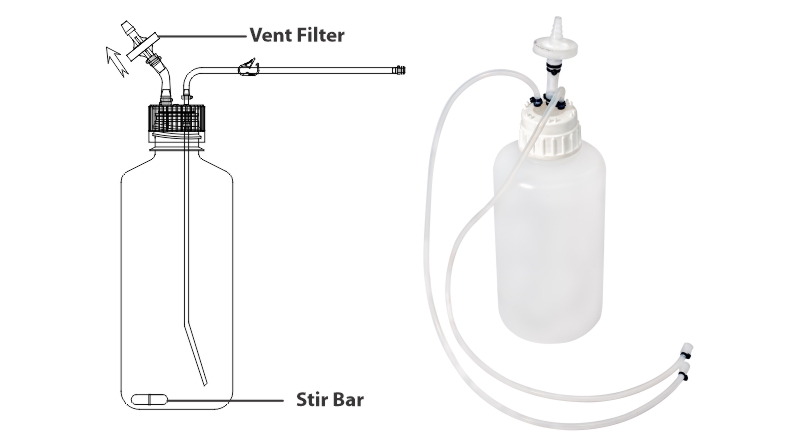
MDI offers AseptiMix VB, customized Bottle Mixer assemblies with PVDF Stir Bar in 500ml, 1 liter, and 2 liters capacities.
Unique Performance Advantages:
- No additional hardware and can directly be placed on a magnetic mixer with a stir bar placed inside.
- PVDF sir bar with wide chemical compatibility and no particle shedding
- With a self supporting lightweight, sterilizing grade 0.2μm PVDF vent filter: Prevents ingress of microorganisms.
Available Sizes:
- 500 ml
- 1 Liter
- 2 Liters
Mixing and transfer applications in:
- Biopharmaceutical product/process development.
- Manufacture of high-value formulations such as radiopharmaceuticals.
Customization:
MDI Vented Bottle Mixer assemblies can be customized to suit user requirements in terms of tubing, fittings, end connections, and connectors.